Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy
- PMID: 36835540
- PMCID: PMC9964041
- DOI: 10.3390/ijms24044131
Spermidine-Eugenol Supplement Preserved Inflammation-Challenged Intestinal Cells by Stimulating Autophagy
Abstract
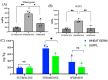
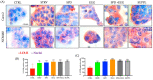
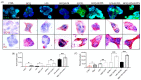
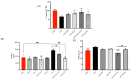
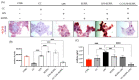
Increases in non-communicable and auto-immune diseases, with a shared etiology of defective autophagy and chronic inflammation, have motivated research both on natural products in drug discovery fields and on the interrelationship between autophagy and inflammation. Within this framework, the tolerability and protective effects of a wheat-germ spermidine (SPD) and clove eugenol (EUG) combination supplement (SUPPL) were investigated on inflammation status (after the administration of lipopolysaccharide (LPS)) and on autophagy using human Caco-2 and NCM460 cell lines. In comparison to the LPS treatment alone, the SUPPL + LPS significantly attenuated ROS levels and midkine expression in monocultures, as well as occludin expression and mucus production in reconstituted intestinal equivalents. Over a timeline of 2-4 h, the SUPPL and SUPPL + LPS treatments stimulated autophagy LC3-11 steady state expression and turnover, as well as P62 turnover. After completely blocking autophagy with dorsomorphin, inflammatory midkine was significantly reduced in the SUPPL + LPS treatment in a non-autophagy-dependent manner. After a 24 h timeline, preliminary results showed that mitophagy receptor BNIP3L expression was significantly downregulated in the SUPPL + LPS treatment compared to the LPS alone, whereas conventional autophagy protein expression was significantly higher. The SUPPL shows promise in reducing inflammation and increasing autophagy to improve intestinal health.
Keywords: Caco-2 cells; LPS; NCM460 cells; autophagy; compound C; eugenol; inflammation; spermidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Naveja J.J., Madariaga-Mazón A., Flores-Murrieta F.J., Granados-Montiel J., Maradiaga-Ceceña M.A., Alaniz V.D., Maldonado-Rodríguez M.R., García-Morales J., Senosiain-Peláez J.P., Martínez-Mayorga K. Union is strength: Antiviral and anti-inflammatory drugs for COVID-19. Drug Discov. Today. 2021;26:29–239. doi: 10.1016/j.drudis.2020.10.018. - DOI - PMC - PubMed
-
- Pereira G., Leão A., Erustes A.G., Morais I., Vrechi T., Zamarioli L., Pereira C., Marchioro L.O., Sperandio L.P., Lins Í., et al. Pharmacological Modulators of Autophagy as a Potential Strategy for the Treatment of COVID-19. Int. J. Mol. Sci. 2021;22:4067. doi: 10.3390/ijms22084067. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

