Caspase Inhibition Modulates Monocyte-Derived Macrophage Polarization in Damaged Tissues
- PMID: 36835566
- PMCID: PMC9964254
- DOI: 10.3390/ijms24044151
Caspase Inhibition Modulates Monocyte-Derived Macrophage Polarization in Damaged Tissues
Abstract
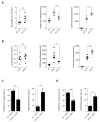
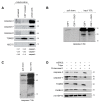
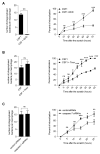
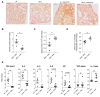
Circulating monocytes are recruited in damaged tissues to generate macrophages that modulate disease progression. Colony-stimulating factor-1 (CSF-1) promotes the generation of monocyte-derived macrophages, which involves caspase activation. Here, we demonstrate that activated caspase-3 and caspase-7 are located to the vicinity of the mitochondria in CSF1-treated human monocytes. Active caspase-7 cleaves p47PHOX at aspartate 34, which promotes the formation of the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase complex NOX2 and the production of cytosolic superoxide anions. Monocyte response to CSF-1 is altered in patients with a chronic granulomatous disease, which are constitutively defective in NOX2. Both caspase-7 down-regulation and radical oxygen species scavenging decrease the migration of CSF-1-induced macrophages. Inhibition or deletion of caspases prevents the development of lung fibrosis in mice exposed to bleomycin. Altogether, a non-conventional pathway that involves caspases and activates NOX2 is involved in CSF1-driven monocyte differentiation and could be therapeutically targeted to modulate macrophage polarization in damaged tissues.
Keywords: CSF1; NOX2; caspase; differentiation; lung fibrosis; macrophage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Hoeffel G., Wang Y., Greter M., See P., Teo P., Malleret B., Leboeuf M., Low D., Oller G., Almeida F., et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

