FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction
- PMID: 36835571
- PMCID: PMC9962088
- DOI: 10.3390/ijms24044159
FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction
Abstract
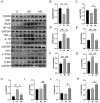
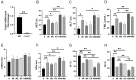
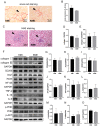
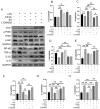
Myocardial infarction (MI) causes peripheral organ injury, in addition to cardiac dysfunction, including in the liver, which is known as cardiac hepatopathy. Aerobic exercise (AE) can effectively improve liver injury, although the mechanism and targets are currently not well established. Irisin, mainly produced by cleavage of the fibronectin type III domain-containing protein 5 (FNDC5), is a responsible for the beneficial effects of exercise training. In this study, we detected the effect of AE on MI-induced liver injury and explored the role of irisin alongside the benefits of AE. Wildtype and Fndc5 knockout mice were used to establish an MI model and subjected to AE intervention. Primary mouse hepatocytes were treated with lipopolysaccharide (LPS), rhirisin, and a phosphoinositide 3-kinase (PI3K) inhibitor. The results showed that AE significantly promoted M2 polarization of macrophages and improved MI-induced inflammation, upregulated endogenous irisin protein expression and activated the PI3K/ protein kinase B (Akt) signaling pathway in the liver of MI mice, while knockout of Fndc5 attenuated the beneficial effects of AE. Exogenous rhirisin significantly inhibited the LPS-induced inflammatory response, which was attenuated by the PI3K inhibitor. These results suggest that AE could effectively activate the FNDC5/irisin-PI3K/Akt signaling pathway, promote the polarization of M2 macrophages, and inhibit the inflammatory response of the liver after MI.
Keywords: aerobic exercise; inflammation; irisin; liver injury; macrophage; myocardial infarction.
Conflict of interest statement
All the authors declare that there are no conflict of interest in this article.
Figures

Similar articles
-
Resistance exercise upregulates Irisin expression and suppresses myocardial fibrosis following myocardial infarction via activating AMPK-Sirt1 and inactivating TGFβ1-Smad2/3.Acta Physiol (Oxf). 2024 Jul;240(7):e14163. doi: 10.1111/apha.14163. Epub 2024 May 16. Acta Physiol (Oxf). 2024. PMID: 38752665
-
Irisin and ALCAT1 mediated aerobic exercise-alleviated oxidative stress and apoptosis in skeletal muscle of mice with myocardial infarction.Free Radic Biol Med. 2022 Nov 20;193(Pt 2):526-537. doi: 10.1016/j.freeradbiomed.2022.10.321. Epub 2022 Nov 3. Free Radic Biol Med. 2022. PMID: 36336228
-
Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway.Free Radic Biol Med. 2020 Oct;158:171-180. doi: 10.1016/j.freeradbiomed.2020.06.038. Epub 2020 Jul 26. Free Radic Biol Med. 2020. PMID: 32726688
-
FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective.Int J Mol Sci. 2021 Feb 5;22(4):1605. doi: 10.3390/ijms22041605. Int J Mol Sci. 2021. PMID: 33562601 Free PMC article. Review.
-
The Role of FNDC5/Irisin in Cardiovascular Disease.Cells. 2024 Feb 2;13(3):277. doi: 10.3390/cells13030277. Cells. 2024. PMID: 38334669 Free PMC article. Review.
Cited by
-
The role and underlying mechanisms of irisin in exercise-mediated cardiovascular protection.PeerJ. 2024 Oct 31;12:e18413. doi: 10.7717/peerj.18413. eCollection 2024. PeerJ. 2024. PMID: 39494293 Free PMC article. Review.
-
Potential role of FNDC5 in exercise-induced improvement of cognitive function.J Zhejiang Univ Sci B. 2025 May 23;26(6):557-572. doi: 10.1631/jzus.B2400016. J Zhejiang Univ Sci B. 2025. PMID: 40571660 Free PMC article. Review.
-
Irisin Alleviates Autoimmune Uveitis Through Promoting Retinal Microglial M1 to M2 Phenotypic Polarization Mediated by HIF-1α Pathway.Inflammation. 2025 Aug;48(4):1716-1727. doi: 10.1007/s10753-024-02149-5. Epub 2024 Sep 29. Inflammation. 2025. PMID: 39342514
-
Physical exercise in liver diseases.Hepatology. 2024 Jun 5:10.1097/HEP.0000000000000941. doi: 10.1097/HEP.0000000000000941. Online ahead of print. Hepatology. 2024. PMID: 38836646
-
The Influence of Irisin on Selected Organs-The Liver, Kidneys, and Lungs: The Role of Physical Exercise.Cells. 2025 Aug 8;14(16):1228. doi: 10.3390/cells14161228. Cells. 2025. PMID: 40862708 Free PMC article. Review.
References
-
- Reinstadler S.J., Reindl M., Feistritzer H.J., Klug G., Mayr A., Kofler M., Tu A.M., Huybrechts L., Mair J., Franz W.M., et al. Prognostic significance of transaminases after acute ST-elevation myocardial infarction: Insights from a cardiac magnetic resonance study. Wien Klin. Wochenschr. 2015;127:843–850. doi: 10.1007/s00508-015-0868-6. - DOI - PubMed
-
- Guzeeva V.A. Liver pathology in myocardial infarct. Kardiologiia. 1977;17:119–123. (In Russian) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

