Treatment of Rapamycin and Evaluation of an Autophagic Response in the Gut of Bactericera cockerelli (Sulč)
- PMID: 36835711
- PMCID: PMC9958837
- DOI: 10.3390/insects14020142
Treatment of Rapamycin and Evaluation of an Autophagic Response in the Gut of Bactericera cockerelli (Sulč)
Abstract
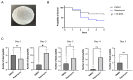
Autophagy is a catabolic process that results in the autophagosomic-lysosomal degradation of bulk cytoplasmic content, abnormal protein aggregates, and excess of/or damaged organelles to promote cell survival. Autophagy is also a component of innate immunity in insects and is involved in the clearance of pathogens, including bacteria. The potato psyllid, Bactericera cockerelli, transmits the plant bacterial pathogen 'Candidatus Liberibacter solanacearum' (Lso) in the Americas and causes serious damage to solanaceous crops. Our previous studies showed that autophagy could be involved in the psyllid response to Lso and could affect pathogen acquisition. However, the tools to evaluate this response have not been validated in psyllids. To this end, the effect of rapamycin, a commonly used autophagy inducer, on potato psyllid survival and the expression of autophagy-related genes was evaluated. Further, the autophagic activity was assessed via microscopy and by measuring the autophagic flux. Artificial diet-feeding assays using rapamycin resulted in significant psyllid mortality, an increase in the autophagic flux, as well as an increase in the amount of autolysosomes. This study represents a stepping stone in determining the role of autophagy in psyllid immunity.
Keywords: autophagy; potato psyllid; programmed cell death; zebra chip; ‘Candidatus Liberibacter solanacearum’.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification of Autophagy-Related Genes in the Potato Psyllid, Bactericera cockerelli and Their Expression Profile in Response to 'Candidatus Liberibacter Solanacearum' in the Gut.Insects. 2021 Nov 30;12(12):1073. doi: 10.3390/insects12121073. Insects. 2021. PMID: 34940161 Free PMC article.
-
'Candidatus Liberibacter solanacearum' Infection of Physalis ixocarpa Brot. (Solanales: Solanaceae) in Saltillo, Mexico.Plant Dis. 2021 Sep;105(9):2560-2566. doi: 10.1094/PDIS-10-20-2240-RE. Epub 2021 Oct 14. Plant Dis. 2021. PMID: 33455443
-
Monitoring and manipulating autophagy in potato psyllids: impacts on accumulation and transmission of "Candidatus Liberibacter solanacearum" haplotypes A and B.Microbiol Spectr. 2025 Aug 19:e0206825. doi: 10.1128/spectrum.02068-25. Online ahead of print. Microbiol Spectr. 2025. PMID: 40827903
-
Biology, Ecology, and Management of the Potato Psyllid, Bactericera cockerelli (Hemiptera: Triozidae), and Zebra Chip Disease in Potato.Annu Rev Entomol. 2024 Jan 25;69:139-157. doi: 10.1146/annurev-ento-020123-014734. Epub 2023 Aug 24. Annu Rev Entomol. 2024. PMID: 37616600 Review.
-
A comprehensive review of zebra chip disease in potato and its management through breeding for resistance/tolerance to 'Candidatus Liberibacter solanacearum' and its insect vector.Pest Manag Sci. 2022 Sep;78(9):3731-3745. doi: 10.1002/ps.6913. Epub 2022 May 6. Pest Manag Sci. 2022. PMID: 35415948 Review.
Cited by
-
Accumulation and Transmission of 'Candidatus Liberibacter solanacearum' Haplotypes by the Nymphs of Two Psyllid Vectors.Insects. 2023 Dec 16;14(12):956. doi: 10.3390/insects14120956. Insects. 2023. PMID: 38132629 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

