Automated Capture of Intraoperative Adverse Events Using Artificial Intelligence: A Systematic Review and Meta-Analysis
- PMID: 36836223
- PMCID: PMC9963108
- DOI: 10.3390/jcm12041687
Automated Capture of Intraoperative Adverse Events Using Artificial Intelligence: A Systematic Review and Meta-Analysis
Abstract
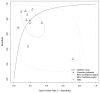
Intraoperative adverse events (iAEs) impact the outcomes of surgery, and yet are not routinely collected, graded, and reported. Advancements in artificial intelligence (AI) have the potential to power real-time, automatic detection of these events and disrupt the landscape of surgical safety through the prediction and mitigation of iAEs. We sought to understand the current implementation of AI in this space. A literature review was performed to PRISMA-DTA standards. Included articles were from all surgical specialties and reported the automatic identification of iAEs in real-time. Details on surgical specialty, adverse events, technology used for detecting iAEs, AI algorithm/validation, and reference standards/conventional parameters were extracted. A meta-analysis of algorithms with available data was conducted using a hierarchical summary receiver operating characteristic curve (ROC). The QUADAS-2 tool was used to assess the article risk of bias and clinical applicability. A total of 2982 studies were identified by searching PubMed, Scopus, Web of Science, and IEEE Xplore, with 13 articles included for data extraction. The AI algorithms detected bleeding (n = 7), vessel injury (n = 1), perfusion deficiencies (n = 1), thermal damage (n = 1), and EMG abnormalities (n = 1), among other iAEs. Nine of the thirteen articles described at least one validation method for the detection system; five explained using cross-validation and seven divided the dataset into training and validation cohorts. Meta-analysis showed the algorithms were both sensitive and specific across included iAEs (detection OR 14.74, CI 4.7-46.2). There was heterogeneity in reported outcome statistics and article bias risk. There is a need for standardization of iAE definitions, detection, and reporting to enhance surgical care for all patients. The heterogeneous applications of AI in the literature highlights the pluripotent nature of this technology. Applications of these algorithms across a breadth of urologic procedures should be investigated to assess the generalizability of these data.
Keywords: PRISMA; adverse effects; artificial intelligence; intraoperative adverse events; intraoperative period; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Quality Assessment of Intraoperative Adverse Event Reporting During 29 227 Robotic Partial Nephrectomies: A Systematic Review and Cumulative Analysis.Eur Urol Oncol. 2020 Dec;3(6):780-783. doi: 10.1016/j.euo.2020.04.003. Epub 2020 May 27. Eur Urol Oncol. 2020. PMID: 32474006
-
Severity Grading Systems for Intraoperative Adverse Events. A Systematic Review of the Literature and Citation Analysis.Ann Surg. 2023 Nov 1;278(5):e973-e980. doi: 10.1097/SLA.0000000000005883. Epub 2023 Apr 27. Ann Surg. 2023. PMID: 37185890
-
Recommendations for Intraoperative Adverse Events Data Collection in Clinical Studies and Study Protocols. An ICARUS Global Surgical Collaboration Study.Int J Surg Protoc. 2023 Feb 9;27(1):23-83. doi: 10.29337/ijsp.183. eCollection 2023. Int J Surg Protoc. 2023. PMID: 36818424 Free PMC article.
-
Intraoperative Adverse Events in Abdominal Surgery: What Happens in the Operating Room Does Not Stay in the Operating Room.Ann Surg. 2017 Jun;265(6):1119-1125. doi: 10.1097/SLA.0000000000001906. Ann Surg. 2017. PMID: 27805961
-
Artificial intelligence for MRI stroke detection: a systematic review and meta-analysis.Insights Imaging. 2024 Jun 24;15(1):160. doi: 10.1186/s13244-024-01723-7. Insights Imaging. 2024. PMID: 38913106 Free PMC article. Review.
Cited by
-
The development of a deep learning model for automated segmentation of the robotic pancreaticojejunostomy.Surg Endosc. 2024 May;38(5):2553-2561. doi: 10.1007/s00464-024-10725-x. Epub 2024 Mar 15. Surg Endosc. 2024. PMID: 38488870
-
Artificial Intelligence Modeling and Priapism.Curr Urol Rep. 2024 Oct;25(10):261-265. doi: 10.1007/s11934-024-01221-9. Epub 2024 Jun 18. Curr Urol Rep. 2024. PMID: 38886246 Review.
-
Artificial Intelligence in Surgery: A Systematic Review of Use and Validation.J Clin Med. 2024 Nov 24;13(23):7108. doi: 10.3390/jcm13237108. J Clin Med. 2024. PMID: 39685566 Free PMC article. Review.
-
Application possibilities of artificial intelligence in facial vascularized composite allotransplantation-a narrative review.Front Surg. 2023 Oct 30;10:1266399. doi: 10.3389/fsurg.2023.1266399. eCollection 2023. Front Surg. 2023. PMID: 38026484 Free PMC article. Review.
-
AI in Surgical Curriculum Design and Unintended Outcomes for Technical Competencies in Simulation Training.JAMA Netw Open. 2023 Sep 5;6(9):e2334658. doi: 10.1001/jamanetworkopen.2023.34658. JAMA Netw Open. 2023. PMID: 37725373 Free PMC article. Clinical Trial.
References
-
- Leape L.L., Brennan T.A., Laird N., Lawthers A.G., Localio A.R., Barnes B.A., Hebert L., Newhouse J.P., Weiler P.C., Hiatt H. The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II. N. Engl. J. Med. 1991;324:377–384. doi: 10.1056/NEJM199102073240605. - DOI - PubMed
-
- Bohnen J.D., Mavros M.N., Ramly E.P., Chang Y., Yeh D.D., Lee J., De Moya M., King D.R., Fagenholz P.J., Butler K. Intraoperative adverse events in abdominal surgery: What happens in the operating room does not stay in the operating room. Ann. Surg. 2017;265:1119–1125. doi: 10.1097/SLA.0000000000001906. - DOI - PubMed
-
- Han K., Bohnen J.D., Peponis T., Martinez M., Nandan A., Yeh D.D., Lee J., Demoya M., Velmahos G., Kaafarani H.M. The surgeon as the second victim? Results of the Boston Intraoperative Adverse Events Surgeons’ Attitude (BISA) study. J. Am. Coll. Surg. 2017;224:1048–1056. doi: 10.1016/j.jamcollsurg.2016.12.039. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

