FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum
- PMID: 36836259
- PMCID: PMC9962196
- DOI: 10.3390/jof9020145
FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum
Abstract
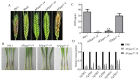
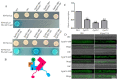
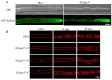
The AP1 complex is a highly conserved clathrin adaptor that plays important roles in regulating cargo protein sorting and intracellular vesicle trafficking in eukaryotes. However, the functions of the AP1 complex in the plant pathogenic fungi including the devastating wheat pathogen Fusarium graminearum are still unclear. In this study, we investigated the biological functions of FgAP1σ, a subunit of the AP1 complex in F. graminearum. Disruption of FgAP1σ causes seriously impaired fungal vegetative growth, conidiogenesis, sexual development, pathogenesis, and deoxynivalenol (DON) production. The ΔFgap1σ mutants were found to be less sensitive to KCl- and sorbitol-induced osmotic stresses but more sensitive to SDS-induced stress than the wild-type PH-1. Although the growth inhibition rate of the ΔFgap1σ mutants was not significantly changed under calcofluor white (CFW) and Congo red (CR) stresses, the protoplasts released from ΔFgap1σ hyphae were decreased compared with the wild-type PH-1, suggesting that FgAP1σ is necessary for cell wall integrity and osmotic stresses in F. graminearum. Subcellular localization assays showed that FgAP1σ was predominantly localized to endosomes and the Golgi apparatus. In addition, FgAP1β-GFP, FgAP1γ-GFP, and FgAP1μ-GFP also localize to the Golgi apparatus. FgAP1β interacts with FgAP1σ, FgAP1γ, and FgAP1μ, while FgAP1σ regulates the expression of FgAP1β, FgAP1γ, and FgAP1μ in F. graminearum. Furthermore, the loss of FgAP1σ blocks the transportation of the v-SNARE protein FgSnc1 from the Golgi to the plasma membrane and delays the internalization of FM4-64 dye into the vacuole. Taken together, our results demonstrate that FgAP1σ plays vital roles in vegetative growth, conidiogenesis, sexual reproduction, DON production, pathogenicity, cell wall integrity, osmotic stress, exocytosis, and endocytosis in F. graminearum. These findings unveil the functions of the AP1 complex in filamentous fungi, most notably in F. graminearum, and lay solid foundations for effective prevention and control of Fusarium head blight (FHB).
Keywords: AP1 complex; FgAP1σ; Fusarium graminearum; wheat scab.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

