Polyketides as Secondary Metabolites from the Genus Aspergillus
- PMID: 36836375
- PMCID: PMC9962652
- DOI: 10.3390/jof9020261
Polyketides as Secondary Metabolites from the Genus Aspergillus
Abstract
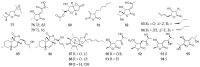
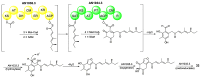
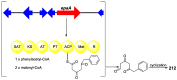
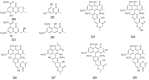
Polyketides are an important class of structurally diverse natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups. These compounds have attracted the worldwide attention of pharmaceutical researchers since they are endowed with a wide array of biological properties. As one of the most common filamentous fungi in nature, Aspergillus spp. is well known as an excellent producer of polyketide compounds with therapeutic potential. By extensive literature search and data analysis, this review comprehensively summarizes Aspergillus-derived polyketides for the first time, regarding their occurrences, chemical structures and bioactivities as well as biosynthetic logics.
Keywords: Aspergillus; bioactivity; fungus; polyketide; secondary metabolite; therapeutic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
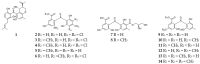
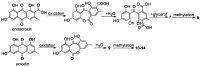
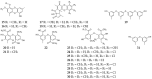
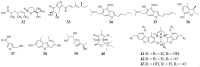

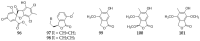
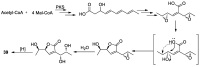

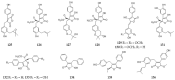
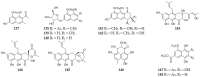


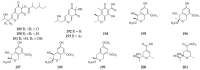
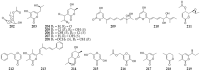

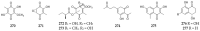
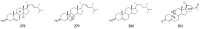


References
-
- Theobald S., Vesth T.C., Rendsvig J.K., Nielsen K.F., Riley R., de Abreu L.M., Salamov A., Frisvad J.C., Larsen T.O., Andersen M.R., et al. Uncovering secondary metabolite evolution and biosynthesis using gene cluster networks and genetic dereplication. Sci. Rep. 2018;8:17957. doi: 10.1038/s41598-018-36561-3. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

