Quantification of Extracellular Volume in CT in Neoadjuvant Chemotherapy in Breast Cancer: New Frontiers in Assessing the Cardiotoxicity of Anthracyclines and Trastuzumab
- PMID: 36836433
- PMCID: PMC9960372
- DOI: 10.3390/jpm13020199
Quantification of Extracellular Volume in CT in Neoadjuvant Chemotherapy in Breast Cancer: New Frontiers in Assessing the Cardiotoxicity of Anthracyclines and Trastuzumab
Abstract
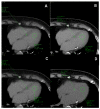
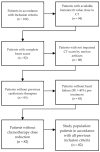
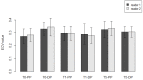
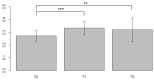
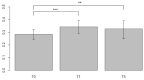
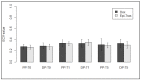
Breast cancer patients undergoing neoadjuvant chemotherapy with anthracyclines or trastuzumab can suffer cardiotoxic issues. Nowadays, the markers of cardiac damage are still not reliable, and extracellular volume (ECV) calculated from CT could be a promising cardiotoxic marker. Eighty-two patients, treated with two different chemotherapy regimens based on doxorubicin (DOX) or epirubicin-trastuzumab (EPI-TRAS), were retrospectively selected and the variations in extracellular volume (ECV) values were measured and analyzed. Whole Body CT (WB-CT) scans were acquired after 1 min, in the portal phase (PP), and after 5 min, in the delayed phases (DP), at the baseline (T0), after one year (T1) and after five years (T5) from the end of chemotherapies. The values measured by two radiologists with different levels of experience were evaluated in order to assess the inter-reader reproducibility assessment (ICC = 0.52 for PP and DP). Further, we performed a population-based analysis and a drug-oriented subgroup analysis in 54 DOX-treated and 28 EPI-TRAS-treated patients. In the general cohort of women treated with any of the two drugs, we observed in the lapse T0-T1 a relative increase (RI) of 25% vs. 20% (PP vs. DP, p < 0.001) as well as in the lapse T0-T5 an RI of 17% vs. 15% (PP vs. DP, p < 0.01). The DOX-treated patients reported in the lapse T0-T1 an RI of 22% (p < 0.0001) in PP and an RI of 16% (p = 0.018) in the DP, with ECV values remaining stably high at T5 both in PP (RI 14.0%, p < 0.0001) and in DP (RI 17%, p = 0.005) highlighting a possible hallmark of a persisting CTX sub-damage. On the other hand, ECV measured in EPI-TRAS-treated women showed an RI in T0-T1 of 18% (p = 0.001) and 29% (p = 0.006) in PP and DP, respectively, but the values returned to basal levels in T5 both in the PP (p = 0.12) and in DP setting (p = 0.13), suggesting damage in the first-year post-treatment and a possible recovery over time. For the 82 patients, an echocardiography was performed at T0, T1= 12 m + 3 m and T5 = 60 m + 6 m with LVEF values at T0 (64% ± 5%), T1 (54% ± 6%) and T5 (53% ± 8%). WB-CT-derived ECV values could provide a valid imaging marker for the early diagnosis of cardiotoxic damage in BC patients undergoing oncological treatments. We detected different patterns during the follow-up, with stably high values for DOX, whereas EPI-TRAS showed a peak within the first year, suggesting different mechanisms of cardiac damage.
Keywords: cardiotoxicity; chemotherapy; extracellular volume.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO Health Organization The Top 10 Causes of Death. [(accessed on 1 February 2022)]. Available online: http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
-
- Orsaria P., Varvaras D., Vanni G., Pagnani G., Scaggiante J., Frusone F., Granai A.V., Petrella G., Buonomo O.C. Nodal Status Assessment in Breast Cancer: Strategies of Clinical Grounds and Quality of Life Implications. Int. J. Breast Cancer. 2014;2014:1–8. doi: 10.1155/2014/469803. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

