Patient Satisfaction and Impact on Oral Health after Maxillary Rehabilitation Using a Personalized Additively Manufactured Subperiosteal Jaw Implant (AMSJI)
- PMID: 36836531
- PMCID: PMC9965586
- DOI: 10.3390/jpm13020297
Patient Satisfaction and Impact on Oral Health after Maxillary Rehabilitation Using a Personalized Additively Manufactured Subperiosteal Jaw Implant (AMSJI)
Abstract
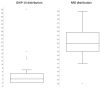
Subperiosteal implants (SIs) were first developed by Dahl in 1941 for oral rehabilitation in case of severe jaw atrophy. Over time, this technique was abandoned due to the high success rate of endosseous implants. The emergence of patient-specific implants and modern dentistry allowed a revisitation of this 80-year-old concept resulting in a novel "high-tech" SI implant. This study evaluates the clinical outcomes in forty patients after maxillary rehabilitation with an additively manufactured subperiosteal jaw implant (AMSJI®). The oral health impact profile-14 (OHIP-14) and numerical rating (NRS) scale were used to assess patient satisfaction and evaluate oral health. In total, fifteen men (mean age: 64.62 years, SD ± 6.75 years) and twenty-five women (mean age: 65.24 years, SD ± 6.77 years) were included, with a mean follow-up time of 917 days (SD ± 306.89 days) after AMSJI installation. Patients reported a mean OHIP-14 of 4.20 (SD ± 7.10) and a mean overall satisfaction based on the NRS of 52.25 (SD ± 4.00). Prosthetic rehabilitation was achieved in all patients. AMSJI is a valuable treatment option for patients with extreme jaw atrophy. Patients enjoy treatment benefits resulting in high patient satisfaction rates and impact on oral health.
Keywords: alveolar bone loss; implant; patient satisfaction; patient-specific implants; subperiosteal; three-dimensional printing.
Conflict of interest statement
Maurice Mommaerts declares that he is an innovation consultant at CADskills BV. All other authors report no conflicts of interest.
Figures


References
-
- Dahl S.G.A. Om möjligheten för implantation i käken av metallskelett som bas eller retention för fasta eller avtagbara protester Särtryck ur Odontologisk Tidskrift häfte. Odontol. Tidskr. 1943;52:440–446.
LinkOut - more resources
Full Text Sources

