The Diagnostic Accuracy of SARS-CoV-2 Nasal Rapid Antigen Self-Test: A Systematic Review and Meta-Analysis
- PMID: 36836639
- PMCID: PMC9961889
- DOI: 10.3390/life13020281
The Diagnostic Accuracy of SARS-CoV-2 Nasal Rapid Antigen Self-Test: A Systematic Review and Meta-Analysis
Abstract
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of coronavirus disease 2019 (COVID-19), a disease that quickly spread into a pandemic. As such, management of the COVID-19 pandemic is deemed necessary, and it can be achieved by using reliable diagnostic tests for SARS-CoV-2. The gold standard for the diagnosis of SARS-CoV-2 is a molecular detection test using the reverse transcription polymerase chain reaction technique (rt-PCR), which is characterized by various disadvantages in contrast with the self-taken nasal rapid antigen tests that produce results faster, have lower costs and do not require specialized personnel. Therefore, the usefulness of self-taken rapid antigen tests is indisputable in disease management, facilitating both the health system and the examinees. Our systematic review aims to access the diagnostic accuracy of the self-taken nasal rapid antigen tests.
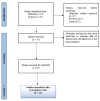
Methods: This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was used to assess the risk of bias in the included studies. All the studies included in this systematic review were found after searching the two databases, Scopus and PubΜed. All but original articles were excluded from this systematic review, while all the studies concerning self-taken rapid antigen tests with a nasal sample and using rt-PCR as a reference test were included. Meta-analysis results and plots were obtained using RevMan software and the MetaDTA website.
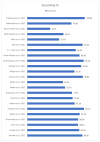
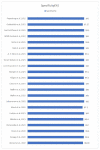
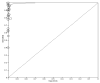
Results: All 22 studies included in this meta-analysis demonstrated a specificity of self-taken rapid antigen tests greater than 98%, which exceeds the minimum required yield for the diagnosis of SARS-CoV-2, according to the WHO. Notwithstanding, the sensitivity varies (from 40% to 98.7%), which makes them in some cases unsuitable for the diagnosis of positive cases. In the majority of the studies, the minimum required performance set by the WHO was achieved, which is 80% compared with rt-PCR tests. The pooled sensitivity of self-taken nasal rapid antigen tests was calculated as 91.1% and the pooled specificity was 99.5%.
Conclusions: In conclusion, self-taken nasal rapid antigen tests have many advantages over rt-PCR tests, such as those related to the rapid reading of the results and their low cost. They also have considerable specificity and some self-taken rapid antigen test kits also have remarkable sensitivity. Consequently, self-taken rapid antigen tests have a wide range of utility but are not able to completely replace rt-PCR tests.
Keywords: SARS-CoV-2; antigen self-test; diagnostic accuracy; rt-PCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Song W.-J., Hui C.K.M., Hull J.H., Birring S.S., McGarvey L., Mazzone S.B., Chung K.F. Confronting COVID-19-associated cough and the post-COVID syndrome: Role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir. Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

