Photocatalytic Synthesis of Materials for Regenerative Medicine Using Complex Oxides with β-pyrochlore Structure
- PMID: 36836711
- PMCID: PMC9959904
- DOI: 10.3390/life13020352
Photocatalytic Synthesis of Materials for Regenerative Medicine Using Complex Oxides with β-pyrochlore Structure
Abstract
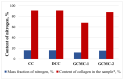
Graft copolymerization of methyl methacrylate onto cod collagen was carried out under visible light irradiation (λ = 400-700 nm) at 20-25 °C using the RbTe1.5W0.5O6, CsTeMoO6, and RbNbTeO6 complex oxides with β-pyrochlore structure as photocatalysts. The as-prepared materials were characterized by X-ray diffraction, scanning electron microscopy, and UV-Vis diffuse reflectance spectroscopy. It was also found that RbNbTeO6 with β-pyrochlore structure was not able to photocatalyze the reaction. Enzymatic hydrolysis of the obtained graft copolymers proceeds with the formation of peptides with a molecular weight (MW) of about 20 and 10 kDa. In contrast to collagen, which decomposes predominantly to peptides with MW of about 10 kDa, the ratio of fractions with MW of about 10 kDa and 20 kDa differs much less, their changes are symbatic, and the content of polymers with MW of more than 20 kDa is about 70% after 1 h in the case of graft copolymers. The data obtained indicate that synthetic fragments grafted to the collagen macromolecule do not prevent the hydrolysis of the peptide bonds but change the rate of polymer degradation. This is important for creating network matrix scaffolds based on graft copolymers by cross-linking peptides, which are products of enzymatic hydrolysis.
Keywords: CsTeMoO6; RbNbTeO6; RbTe1.5W0.5O6; cod collagen; complex oxides; enzymatic hydrolysis; graft copolymer; methyl methacrylate; photocatalysis; scaffold; β-pyrochlores.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Antibacterial effect of new photocatalytically active "complex oxides/PMMA" composites under visible-light irradiation.Photochem Photobiol Sci. 2024 Dec;23(12):2237-2253. doi: 10.1007/s43630-024-00664-w. Epub 2024 Nov 29. Photochem Photobiol Sci. 2024. PMID: 39612098
-
Enzymatic Hydrolysis of Marine Collagen and Fibrinogen Proteins in the Presence of Thrombin.Mar Drugs. 2020 Apr 11;18(4):208. doi: 10.3390/md18040208. Mar Drugs. 2020. PMID: 32290502 Free PMC article.
-
Copper-Modified Titania-Based Photocatalysts for the Efficient Hydrogen Production under UV and Visible Light from Aqueous Solutions of Glycerol.Nanomaterials (Basel). 2022 Sep 7;12(18):3106. doi: 10.3390/nano12183106. Nanomaterials (Basel). 2022. PMID: 36144894 Free PMC article.
-
Construction of hierarchical nanostructured TiO2/Bi2MoO6 heterojunction for improved visible light photocatalysis.J Nanosci Nanotechnol. 2012 Aug;12(8):6294-300. doi: 10.1166/jnn.2012.6200. J Nanosci Nanotechnol. 2012. PMID: 22962739
-
Scaffold Chemical Model Based on Collagen-Methyl Methacrylate Graft Copolymers.Polymers (Basel). 2023 Jun 8;15(12):2618. doi: 10.3390/polym15122618. Polymers (Basel). 2023. PMID: 37376264 Free PMC article.
Cited by
-
Production of Graft Copolymers of Cod Collagen with Butyl Acrylate and Vinyl Butyl Ether in the Presence of Triethylborane-Prospects for Use in Regenerative Medicine.Polymers (Basel). 2023 Jul 25;15(15):3159. doi: 10.3390/polym15153159. Polymers (Basel). 2023. PMID: 37571053 Free PMC article.
-
Biocompatibility of New Hydrogels Based on a Copolymer of Fish Collagen and Methyl Methacrylate Obtained Using Heterogeneous Photocatalysis Under the Influence of Visible Light.Polymers (Basel). 2025 Jul 22;17(15):2002. doi: 10.3390/polym17152002. Polymers (Basel). 2025. PMID: 40808051 Free PMC article.
References
-
- Alrahlah A., Khan R., Vohra F., Alqahtani I.M., Alruhaymi A.A., Haider S., Al-Odayni A.-B.M., Saeed W.S., Ananda Murthy H.C., Bautista L. Influence of the physical inclusion of ZrO2/TiO2 nanoparticles on physical, mechanical, and morphological characteristics of PMMA based interim restorative material. BioMed Res. Int. 2022;2022:1743019. doi: 10.1155/2022/1743019. - DOI - PMC - PubMed
-
- Anil Kumar M.R., Abebe B., Nagaswarupa H.P., Ananda Murthy H.C., Ravikumar C.R., Sabir F.K. Enhanced photocatalytic and electrochemical performance of TiO2/Fe2O3 Nanocomposites; its application in dye decolorization and supercapacitors. Sci. Rep. Nat. J. 2020;10:1249. doi: 10.1038/s41598-020-58110-7. - DOI - PMC - PubMed
-
- Rosman N.N., Yunus R.M., Mohd Shah N.R.A., Mohd Shah R., Arifin K., Minggu L.J., Ludin N.A. An overview of co-catalysts on metal oxides for photocatalytic water splitting. Int. J. Energy Res. 2022;46:11596. doi: 10.1002/er.8001. - DOI
-
- Kobayashi A., Takizawa S., Hirahara M. Photofunctional molecular assembly for artificial photosynthesis: Beyond a simple dye sensitization strategy. Coord. Chem. Rev. 2022;467:214624. doi: 10.1016/j.ccr.2022.214624. - DOI
LinkOut - more resources
Full Text Sources

