Spark of Life: Role of Electrotrophy in the Emergence of Life
- PMID: 36836714
- PMCID: PMC9961546
- DOI: 10.3390/life13020356
Spark of Life: Role of Electrotrophy in the Emergence of Life
Abstract
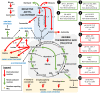
The emergence of life has been a subject of intensive research for decades. Different approaches and different environmental "cradles" have been studied, from space to the deep sea. Since the recent discovery of a natural electrical current through deep-sea hydrothermal vents, a new energy source is considered for the transition from inorganic to organic. This energy source (electron donor) is used by modern microorganisms via a new trophic type, called electrotrophy. In this review, we draw a parallel between this metabolism and a new theory for the emergence of life based on this electrical electron flow. Each step of the creation of life is revised in the new light of this prebiotic electrochemical context, going from the evaluation of similar electrical current during the Hadean, the CO2 electroreduction into a prebiotic primordial soup, the production of proto-membranes, the energetic system inspired of the nitrate reduction, the proton gradient, and the transition to a planktonic proto-cell. Finally, this theory is compared to the two other theories in hydrothermal context to assess its relevance and overcome the limitations of each. Many critical factors that were limiting each theory can be overcome given the effect of electrochemical reactions and the environmental changes produced.
Keywords: electroreduction of CO2; electrotrophy; emergence of life; hydrothermal vents; prebiotic synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The Origin of Life in Alkaline Hydrothermal Vents.Astrobiology. 2016 Feb;16(2):181-97. doi: 10.1089/ast.2015.1406. Epub 2016 Feb 3. Astrobiology. 2016. PMID: 26841066 Review.
-
Prebiotic Formation of Protoalkaloids within Alkaline Oceanic Hydrothermal Vents in the Hadean Seafloor as a Prerequisite for Evolutionary Biodiversity.Med Sci Monit. 2020 Sep 22;26:e928415. doi: 10.12659/MSM.928415. Med Sci Monit. 2020. PMID: 32959807 Free PMC article.
-
Natural pH Gradients in Hydrothermal Alkali Vents Were Unlikely to Have Played a Role in the Origin of Life.J Mol Evol. 2016 Aug;83(1-2):1-11. doi: 10.1007/s00239-016-9756-6. Epub 2016 Aug 17. J Mol Evol. 2016. PMID: 27534947 Free PMC article. Review.
-
CO2 reduction driven by a pH gradient.Proc Natl Acad Sci U S A. 2020 Sep 15;117(37):22873-22879. doi: 10.1073/pnas.2002659117. Epub 2020 Sep 8. Proc Natl Acad Sci U S A. 2020. PMID: 32900930 Free PMC article.
-
A symbiotic view of the origin of life at hydrothermal impact crater-lakes.Phys Chem Chem Phys. 2016 Jul 27;18(30):20033-46. doi: 10.1039/c6cp00550k. Phys Chem Chem Phys. 2016. PMID: 27126878
References
-
- Gesteland R., Atkins J. The RNA World. Cold Spring Harbor Laboratory Press; Long Island, NY, USA: 1993.
Publication types
LinkOut - more resources
Full Text Sources

