Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins
- PMID: 36836723
- PMCID: PMC9962328
- DOI: 10.3390/life13020367
Arachidonic Acid Added during the Differentiation Phase of 3T3-L1 Cells Exerts Anti-Adipogenic Effect by Reducing the Effects of Pro-Adipogenic Prostaglandins
Abstract
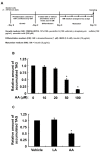
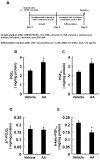
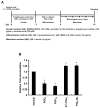
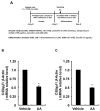
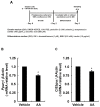
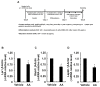
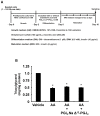
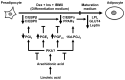
A linoleic acid (LA) metabolite arachidonic acid (AA) added to 3T3-L1 cells is reported to suppress adipogenesis. The purpose of the present study aimed to clarify the effects of AA added during the differentiation phase, including adipogenesis, the types of prostaglandins (PG)s produced, and the crosstalk between AA and the PGs produced. Adipogenesis was inhibited by AA added, while LA did not. When AA was added, increased PGE2 and PGF2α production, unchanged Δ12-PGJ2 production, and reduced PGI2 production were observed. Since the decreased PGI2 production was reflected in decreased CCAAT/enhancer-binding protein-β (C/EBPβ) and C/EBPδ expression, we expected that the coexistence of PGI2 with AA would suppress the anti-adipogenic effects of AA. However, the coexistence of PGI2 with AA did not attenuate the anti-adipogenic effects of AA. In addition, the results were similar when Δ12-PGJ2 coexisted with AA. Taken together, these results indicated that the metabolism of ingested LA to AA is necessary to inhibit adipogenesis and that exposure of AA to adipocytes during only the differentiation phase is sufficient. As further mechanisms for suppressing adipogenesis, AA was found not only to increase PGE2 and PGF2α and decrease PGI2 production but also to abrogate the pro-adipogenic effects of PGI2 and Δ12-PGJ2.
Keywords: 6-PUFAs; adipogenesis; arachidonic acid; prostaglandin; the differentiation phase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Green H., Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–116. doi: 10.1016/0092-8674(74)90126-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

