Effect of Abscisic Acid on Growth, Fatty Acid Profile, and Pigment Composition of the Chlorophyte Chlorella (Chromochloris) zofingiensis and Its Co-Culture Microbiome
- PMID: 36836809
- PMCID: PMC9962398
- DOI: 10.3390/life13020452
Effect of Abscisic Acid on Growth, Fatty Acid Profile, and Pigment Composition of the Chlorophyte Chlorella (Chromochloris) zofingiensis and Its Co-Culture Microbiome
Abstract
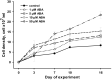
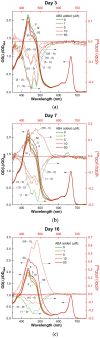
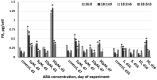
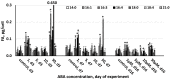
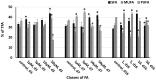
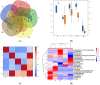
Microalga Chlorella (Chromochloris) zofingiensis has been gaining increasing attention of investigators as a potential competitor to Haematococcus pluvialis for astaxanthin and other xanthophylls production. Phytohormones, including abscisic acid (ABA), at concentrations relevant to that in hydroponic wastewater, have proven themselves as strong inductors of microalgae biomass productivity and biosynthesis of valuable molecules. The main goal of this research was to evaluate the influence of phytohormone ABA on the physiology of C. zofingiensis in a non-aseptic batch experiment. Exogenous ABA stimulated C. zofingiensis cell division, biomass production, as well as chlorophyll, carotenoid, and lipid biosynthesis. The relationship between exogenous ABA concentration and the magnitude of the observed effects was non-linear, with the exception of cell growth and biomass production. Fatty acid accumulation and composition depended on the concentration of ABA tested. Exogenous ABA induced spectacular changes in the major components of the culture microbiome of C. zofingiensis. Thus, the abundance of the representatives of the genus Rhodococcus increased drastically with an increase in ABA concentration, whereas the abundance of the representatives of Reyranella and Bradyrhizobium genera declined. The possibilities of exogenous ABA applications for the enhancing of the biomass, carotenoid, and fatty acid productivity of the C. zofingiensis cultures are discussed.
Keywords: bacterial community; carotenoids; chlorophyll; fatty acid profile; growth parameters; microalgae; phytohormone.
Conflict of interest statement
The authors declare no conflict of interest to disclose. No conflict, informed consent, human or animal rights applicable.
Figures
References
-
- Harwood J.L., Jones A.L. Lipid metabolism in algae. In: Callow J.A., editor. Advances in Botanical Research. Volume 16. Academic Press; Waltham, MA, USA: 1989. pp. 1–53. - DOI
-
- Solovchenko A., Khozin-Goldberg I., Didi-Cohen S., Cohen Z.R., Merzlyak M.N. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga. J. Appl. Phycol. 2007;43:833–843. doi: 10.1007/s10811-007-9233-0. - DOI
-
- Babu A.G., Wu X., Kabra A.N., Kim D.P. Cultivation of an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitation. Algal Res. 2017;23:178–185. doi: 10.1016/j.algal.2017.02.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

