Effects of Pirfenidone on Idiopathic Pulmonary Fibrosis Progression and Safety: Results of Multicenter Prospective Observational Study
- PMID: 36836840
- PMCID: PMC9963853
- DOI: 10.3390/life13020483
Effects of Pirfenidone on Idiopathic Pulmonary Fibrosis Progression and Safety: Results of Multicenter Prospective Observational Study
Abstract
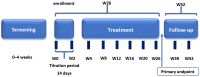
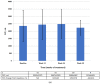
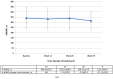
The aim of this study was to determine the effectiveness of pirfenidone in patients with idiopathic pulmonary fibrosis (IPF) seen in clinical practice. Fifty-five adults with IPF were enrolled in this multicenter, open-label, non-randomized, non-controlled, interventional clinical study. All patients received pirfenidone 2403 mg/day (three 267 mg capsules three times daily) for 26 weeks. After 26 weeks of treatment, the mean change in absolute forced vital capacity (FVC) was 128.8 mL (95% confidence interval [CI] -26.8, 284.4) and the mean change in relative predicted FVC was -0.10% (95% CI -3.18, 2.99). Stable disease (defined as improvement of ≥0% or a decline of <10% to 0% of the corresponding FVC value) was observed in most patients (relative FVC, 90.9%; absolute FVC, 83.6%). There was no statistically significant change in the mean high-resolution computed tomography fibrosis score or lung opacity score at week 26 compared with baseline. Treatment-emergent adverse events were reported in 80% of patients during the treatment period; most of them were mild or moderate in severity. No serious pirfenidone-related adverse events were observed during the study period. Pirfenidone was generally safe and effective for controlling functional decline and stabilizing disease in patients with IPF encountered in clinical practice in Russia.
Keywords: idiopathic pulmonary fibrosis; pirfenidone; progression; safety.
Conflict of interest statement
F. Hoffmann-La Roche Ltd. designed the study protocol and conducted and monitored the study in accordance with the standard operating procedure for clinical studies, and generated a clinical study report. The study interpretation, writing of the manuscript, and decision to publish the manuscript were the sole responsibility of the authors and independent of the funders. Otherwise, the authors have no conflicts of interest to declare regarding the content of this article.
Figures



References
-
- Wolters P.J., Blackwell T.S., Eickelberg O., Loyd J.E., Kaminski N., Jenkins G., Maher T.M., Molina-Molina M., Noble P.W., Raghu G., et al. Time for a change: Is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

