Exploring the Role of ACE2 as a Connecting Link between COVID-19 and Parkinson's Disease
- PMID: 36836893
- PMCID: PMC9961012
- DOI: 10.3390/life13020536
Exploring the Role of ACE2 as a Connecting Link between COVID-19 and Parkinson's Disease
Abstract
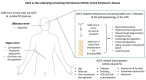
Coronavirus disease 2019 (COVID-19) is frequently accompanied by neurological manifestations such as headache, delirium, and epileptic seizures, whereas ageusia and anosmia may appear before respiratory symptoms. Among the various neurological COVID-19-related comorbidities, Parkinson's disease (PD) has gained increasing attention. Some cases of PD disease have been linked to COVID-19, and both motor and non-motor symptoms in Parkinson's disease patients frequently worsen following SARS-CoV-2 infection. Although it is still unclear whether PD increases the susceptibility to SARS-CoV-2 infection or whether COVID-19 increases the risk of or unmasks future cases of PD, emerging evidence sheds more light on the molecular mechanisms underlying the relationship between these two diseases. Among them, angiotensin-converting enzyme 2 (ACE2), a significant component of the renin-angiotensin system (RAS), seems to play a pivotal role. ACE2 is required for the entry of SARS-CoV-2 to the human host cells, and ACE2 dysregulation is implicated in the severity of COVID-19-related acute respiratory distress syndrome (ARDS). ACE2 imbalance is implicated in core shared pathophysiological mechanisms between PD and COVID-19, including aberrant inflammatory responses, oxidative stress, mitochondrial dysfunction, and immune dysregulation. ACE2 may also be implicated in alpha-synuclein-induced dopaminergic degeneration, gut-brain axis dysregulation, blood-brain axis disruption, autonomic dysfunction, depression, anxiety, and hyposmia, which are key features of PD.
Keywords: COVID-19 pandemic; dopaminergic degeneration; neurodegeneration; neuroinflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson's and Alzheimer's Disease.Oxid Med Cell Longev. 2022 Aug 31;2022:3012778. doi: 10.1155/2022/3012778. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092161 Free PMC article. Review.
-
The Influence of Coronavirus Disease-2019 (COVID-19) On Parkinson's Disease: An Updated Systematic Review.J Prim Care Community Health. 2021 Jan-Dec;12:21501327211039709. doi: 10.1177/21501327211039709. J Prim Care Community Health. 2021. PMID: 34404266 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Role of Inflammation in the Development of COVID-19 to Parkinson's Disease.J Inflamm Res. 2024 May 21;17:3259-3282. doi: 10.2147/JIR.S460161. eCollection 2024. J Inflamm Res. 2024. PMID: 38800597 Free PMC article.
-
Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update.High Blood Press Cardiovasc Prev. 2021 Mar;28(2):129-139. doi: 10.1007/s40292-021-00439-9. Epub 2021 Feb 26. High Blood Press Cardiovasc Prev. 2021. PMID: 33635533 Free PMC article. Review.
Cited by
-
Differential Roles of Angiotensin A and Alamandine in Parkinson's Disease: A Therapeutic Perspective on Nonclassical RAS Pathways.Brain Behav. 2025 Aug;15(8):e70721. doi: 10.1002/brb3.70721. Brain Behav. 2025. PMID: 40791030 Free PMC article. Review.
-
New‑onset non‑motor symptoms in patients with Parkinson's disease and post‑COVID‑19 syndrome: A prospective cross‑sectional study.Med Int (Lond). 2023 Apr 11;3(3):23. doi: 10.3892/mi.2023.83. eCollection 2023 May-Jun. Med Int (Lond). 2023. PMID: 37214229 Free PMC article.
-
The Possible Mechanistic Basis of Individual Susceptibility to Spike Protein Injury.Adv Virol. 2025 Jun 24;2025:7990876. doi: 10.1155/av/7990876. eCollection 2025. Adv Virol. 2025. PMID: 40599824 Free PMC article. Review.
-
Parkinson's Disease, SARS-CoV-2, and Frailty: Is There a Vicious Cycle Related to Hypovitaminosis D?Brain Sci. 2023 Mar 23;13(4):528. doi: 10.3390/brainsci13040528. Brain Sci. 2023. PMID: 37190492 Free PMC article. Review.
-
Characteristics and outcomes of elderly patients with Parkinson's disease hospitalized due to COVID‑19‑associated pneumonia.Med Int (Lond). 2023 Jul 4;3(4):34. doi: 10.3892/mi.2023.94. eCollection 2023 Jul-Aug. Med Int (Lond). 2023. PMID: 37448768 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

