Synthesis of Micro- and Mesoporous Carbon Foams with Nanodispersed Metals for Adsorption and Catalysis Applications
- PMID: 36836966
- PMCID: PMC9963879
- DOI: 10.3390/ma16041336
Synthesis of Micro- and Mesoporous Carbon Foams with Nanodispersed Metals for Adsorption and Catalysis Applications
Abstract
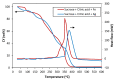
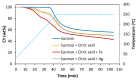
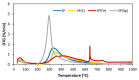
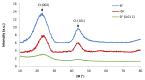
This work focuses on carbon foams, whose peculiarity is a predominant open macroporous cellular network that can be provided with tailored texture and morphology by the modification of the preparation process. The goal was to obtain macroporous carbonaceous structures capable of being activated by following a simple thermo-foaming procedure using a few reagents. With this purpose in mind, carbon foams with different textural properties were synthesized from sucrose using two foaming processes: at atmospheric pressure and in a pressurized reactor. Iron and silver nitrates added to sucrose gave rise, after carbonization, to materials with iron oxides and elemental silver particles nano-dispersed in the carbon matrix and promoted microporosity in both cases and mesoporosity in the case of iron nitrate. Iron nitrate also catalyzes the graphitization of the carbon material during carbonization. All these findings show the potential of sucrose thermo-foaming process as a viable and sustainable path to produce versatile carbon materials, capable of being used in various applications.
Keywords: carbon foams; metal nitrates; sucrose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Inagaki H., Kang M., Toyoda F., Konno M. Advanced Materials Science and Engineering of Carbon. Elsevier; Amsterdam, The Netherlands: 2013. [(accessed on 31 January 2023)]. Introduction; pp. 1–13. Available online: https://www.elsevier.com/books/advanced-materials-science-and-engineerin....
-
- Inagaki M., Qiu J., Guo Q. Carbon foam: Preparation and application. Carbon. 2015;87:128–152. doi: 10.1016/j.carbon.2015.02.021. - DOI
-
- Stojanovska E., Calisir M.D., Ozturk N.D., Kilic A. Carbon-Based Foams: Preparation and Applications. Elsevier Ltd.; Amsterdam, The Netherlands: 2018. - DOI
-
- Fernández-Miranda N., Rodríguez E., Lopez-Anton M.A., García R., Martínez-Tarazona M.R. A new approach for retaining mercury in energy generation processes: Regenerable carbonaceous sorbents. Energies. 2017;10:1311. doi: 10.3390/en10091311. - DOI
-
- Antuña-Nieto C., Rodríguez E., Lopez-Anton M., García R., Martínez-Tarazona M. A candidate material for mercury control in energy production processes: Carbon foams loaded with gold. Energy. 2018;159:630–637. doi: 10.1016/j.energy.2018.06.172. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

