Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time
- PMID: 36837301
- PMCID: PMC9963818
- DOI: 10.3390/ma16041672
Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time
Abstract
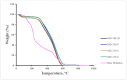
In this work, carbon microspheres (CMs) were prepared by hydrothermal carbonization (HTC) of inedible crystallized date palm molasses. The effects of temperature and reaction time on the prepared materials were studied. Experiments were carried out at different temperatures (180, 200, 230 and 250 °C) with reaction times ranging from 2 to 10 h. It was found that temperature had the greatest influence on the mass yield of the CMs. No solid products were observed at a temperature of 180 °C and a reaction time less than 2 h. The highest yield was found to be 40.4% at 250 °C and a reaction time of 6 h. The results show that the CMs produced were approximately 5-9 μm in diameter. The results also show that the largest diameter of the CMs (8.9 μm) was obtained at a temperature of 250 °C and a reaction time of 6 h. Nonetheless, if the reaction time was extended beyond 6 h at 250 °C, the CMs fused and their shapes were deformed (non-spherical shapes). The synthesized materials were characterized using Scanning Electron Microscopy (SEM), Fourier Transform Infrared (FTIR), Branuer-Emmett-Teller (BET) and thermogravimetric analysis (TGA). BET surface areas for the four samples were found to be less than 1 m2/g. The methylene blue adsorption studies indicated that the equilibrium adsorption capacity was reached after 15 min, with a maximum adsorption capacity of 12 mg/g. The recycling of date palm molasses (a known processed waste) to generate a useable carbon microsphere represents a beneficial step in the application of sustainable processing industries in the Middle East.
Keywords: carbon microspheres; hydrothermal carbonization; inedible crystallized date palm molasses; reaction time; temperature; yield.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Abbas A., Mariana L.T., Phan A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon. 2018;140:77–99. doi: 10.1016/j.carbon.2018.08.016. - DOI
-
- Titirici M.M., Antonietti M., Baccile N. Hydrothermal carbon from biomass: A comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem. 2008;10:1204–1212. doi: 10.1039/b807009a. - DOI
-
- Sevilla M., Fuertes A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon. 2009;47:2281–2289. doi: 10.1016/j.carbon.2009.04.026. - DOI
-
- Cai H., Lin X., Qin Y., Luo X. Hydrothermal synthesis of carbon microsphere from glucose at low temperature and its adsorption property of uranium (VI) J. Radioanal. Nucl. Chem. 2017;311:695–706. doi: 10.1007/s10967-016-5106-9. - DOI
LinkOut - more resources
Full Text Sources

