Odor Discrimination by Lipid Membranes
- PMID: 36837654
- PMCID: PMC9962961
- DOI: 10.3390/membranes13020151
Odor Discrimination by Lipid Membranes
Abstract
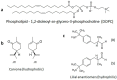
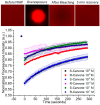
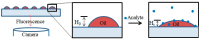
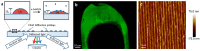
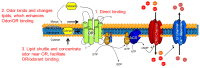
Odor detection and discrimination in mammals is known to be initiated by membrane-bound G-protein-coupled receptors (GPCRs). The role that the lipid membrane may play in odor discrimination, however, is less well understood. Here, we used model membrane systems to test the hypothesis that phospholipid bilayer membranes may be capable of odor discrimination. The effect of S-carvone, R-carvone, and racemic lilial on the model membrane systems was investigated. The odorants were found to affect the fluidity of supported lipid bilayers as measured by fluorescence recovery after photobleaching (FRAP). The effect of odorants on surface-supported lipid multilayer microarrays of different dimensions was also investigated. The lipid multilayer micro- and nanostructure was highly sensitive to exposure to these odorants. Fluorescently-labeled lipid multilayer droplets of 5-micron diameter were more responsive to these odorants than ethanol controls. Arrays of lipid multilayer diffraction gratings distinguished S-carvone from R-carvone in an artificial nose assay. Our results suggest that lipid bilayer membranes may play a role in odorant discrimination and molecular recognition in general.
Keywords: biosensor; droplet; enantioselectivity; lipid; lithography; microarray; nanointaglio; nose; odorant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

