An Isotonic Model of Neuron Swelling Based on Co-Transport of Salt and Water
- PMID: 36837709
- PMCID: PMC9958824
- DOI: 10.3390/membranes13020206
An Isotonic Model of Neuron Swelling Based on Co-Transport of Salt and Water
Abstract
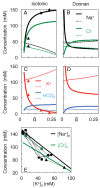
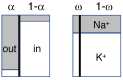
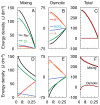
Neurons spend most of their energy building ion gradients across the cell membrane. During energy deprivation the neurons swell, and the concomitant mixing of their ions is commonly assumed to lead toward a Donnan equilibrium, at which the concentration gradients of all permeant ion species have the same Nernst potential. This Donnan equilibrium, however, is not isotonic, as the total concentration of solute will be greater inside than outside the neurons. The present theoretical paper, in contrast, proposes that neurons follow a path along which they swell quasi-isotonically by co-transporting water and ions. The final neuronal volume on the path is taken that at which the concentration of impermeant anions in the shrinking extracellular space equals that inside the swelling neurons. At this final state, which is also a Donnan equilibrium, all permeant ions can mix completely, and their Nernst potentials vanish. This final state is isotonic and electro-neutral, as are all intermediate states along this path. The path is in principle reversible, and maximizes the work of mixing.
Keywords: KCC2; brain; co-transporter; concentration gradient; energy; extracellular space; ions; ischemia; mixing; osmosis.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

