Remodeling of Biomembranes and Vesicles by Adhesion of Condensate Droplets
- PMID: 36837726
- PMCID: PMC9965763
- DOI: 10.3390/membranes13020223
Remodeling of Biomembranes and Vesicles by Adhesion of Condensate Droplets
Abstract
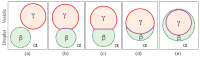
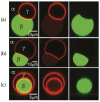
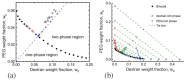
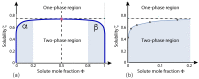
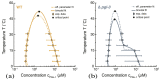
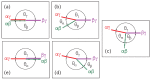
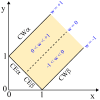
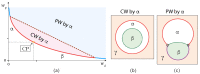
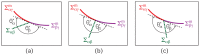
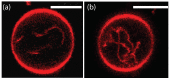
Condensate droplets are formed in aqueous solutions of macromolecules that undergo phase separation into two liquid phases. A well-studied example are solutions of the two polymers PEG and dextran which have been used for a long time in biochemical analysis and biotechnology. More recently, phase separation has also been observed in living cells where it leads to membrane-less or droplet-like organelles. In the latter case, the condensate droplets are enriched in certain types of proteins. Generic features of condensate droplets can be studied in simple binary mixtures, using molecular dynamics simulations. In this review, I address the interactions of condensate droplets with biomimetic and biological membranes. When a condensate droplet adheres to such a membrane, the membrane forms a contact line with the droplet and acquires a very high curvature close to this line. The contact angles along the contact line can be observed via light microscopy, lead to a classification of the possible adhesion morphologies, and determine the affinity contrast between the two coexisting liquid phases and the membrane. The remodeling processes generated by condensate droplets include wetting transitions, formation of membrane nanotubes as well as complete engulfment and endocytosis of the droplets by the membranes.
Keywords: adhesion; biomembranes; condensate droplets; endocytosis; engulfment; line tension; membrane tubulation; surface tensions; synthetic biosystems; wetting transitions.
Conflict of interest statement
The author declares no conflict of interest.
Figures

















References
-
- Bassereau P., Sens P., editors. Physics of Biological Membranes. Springer Nature; Cham, Switzerland: 2018.
-
- Dimova R., Marques C., editors. The Giant Vesicle Book. Taylor & Francis; Boca Raton, FL, USA: 2020.
-
- Young T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805;95:65–87.
Publication types
LinkOut - more resources
Full Text Sources

