Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata
- PMID: 36837908
- PMCID: PMC9963190
- DOI: 10.3390/metabo13020289
Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata
Abstract
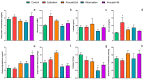
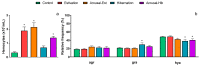
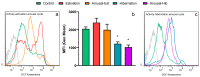
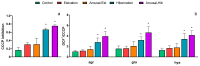
States of natural dormancy include estivation and hibernation. Ampullariids are exemplary because they undergo estivation when deprived of water or hibernation when exposed to very low temperatures. Regardless of the condition, ampullariids show increased endogenous antioxidant defenses, anticipating the expected respiratory burst during reoxygenation after reactivation, known as "Preparation for Oxidative Stress (POS)". In this work, we tested the POS hypothesis for changes in the blood and hemocytes of the bimodal breather Pomacea canaliculata (Ampullariidae) induced at experimental estivation and hibernation. We described respiratory (hemocyanin, proteins, lactate), antioxidant (GSH, uric acid, SOD, CAT, GST), and immunological (hemocyte levels, ROS production) parameters. We showed that, although the protein level remains unchanged in all experimental groups, hemocyanin increases in response to estivation. Furthermore, lactate remains unchanged in challenged snails, suggesting an aerobic metabolism during short-term challenges. Blood uric acid increases during estivation and arousal from estivation or hibernation, supporting the previously proposed antioxidant role. Regarding hemocytes, we showed that the total population increases with all challenges, and granulocytes increase during hibernation. We further showed that hibernation affects ROS production by hemocytes, possibly through mitochondrial inhibition. This study contributed to the knowledge of the adaptive strategies of ampullariids to tolerate adverse environmental conditions.
Keywords: Oxidative stress defenses; hemocyanin; hypometabolism; immune system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antioxidant Responses Induced by Short-Term Activity-Estivation-Arousal Cycle in Pomacea canaliculata.Front Physiol. 2022 Feb 2;13:805168. doi: 10.3389/fphys.2022.805168. eCollection 2022. Front Physiol. 2022. PMID: 35185614 Free PMC article.
-
Tolerance to hypometabolism and arousal induced by hibernation in the apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae).Comp Biochem Physiol B Biochem Mol Biol. 2018 Oct;224:129-137. doi: 10.1016/j.cbpb.2017.12.015. Epub 2017 Dec 19. Comp Biochem Physiol B Biochem Mol Biol. 2018. PMID: 29277604
-
Uric acid deposits and estivation in the invasive apple-snail, Pomacea canaliculata.Comp Biochem Physiol A Mol Integr Physiol. 2011 Apr;158(4):506-12. doi: 10.1016/j.cbpa.2010.12.012. Epub 2010 Dec 21. Comp Biochem Physiol A Mol Integr Physiol. 2011. PMID: 21182978
-
Antioxidant defenses and metabolic depression. The hypothesis of preparation for oxidative stress in land snails.Comp Biochem Physiol B Biochem Mol Biol. 1998 Jul;120(3):437-48. doi: 10.1016/s0305-0491(98)10053-6. Comp Biochem Physiol B Biochem Mol Biol. 1998. PMID: 9787804 Review.
-
Life in the slow lane: molecular mechanisms of estivation.Comp Biochem Physiol A Mol Integr Physiol. 2002 Nov;133(3):733-54. doi: 10.1016/s1095-6433(02)00206-4. Comp Biochem Physiol A Mol Integr Physiol. 2002. PMID: 12443930 Review.
Cited by
-
Changes in the oxidative status and damage by non-essential elements in the digestive gland of the gastropod Pomacea canaliculata.Front Physiol. 2023 Mar 22;14:1123977. doi: 10.3389/fphys.2023.1123977. eCollection 2023. Front Physiol. 2023. PMID: 37035656 Free PMC article.
-
Glutathione Depletion Disrupts Redox Homeostasis in an Anoxia-Tolerant Invertebrate.Antioxidants (Basel). 2023 May 31;12(6):1197. doi: 10.3390/antiox12061197. Antioxidants (Basel). 2023. PMID: 37371926 Free PMC article.
-
Dynamics in gut microbiota diversity, composition, and assembly reveal the adaptability of invasive snail Pomacea canaliculata during hibernation in rice fields.Front Microbiol. 2025 Jul 16;16:1616681. doi: 10.3389/fmicb.2025.1616681. eCollection 2025. Front Microbiol. 2025. PMID: 40740324 Free PMC article.
-
The role of solar radiation and tidal emersion on oxidative stress and glutathione synthesis in mussels exposed to air.PeerJ. 2023 May 11;11:e15345. doi: 10.7717/peerj.15345. eCollection 2023. PeerJ. 2023. PMID: 37193036 Free PMC article.
-
The Eco-Immunological Relevance of the Anti-Oxidant Response in Invasive Molluscs.Antioxidants (Basel). 2023 Jun 13;12(6):1266. doi: 10.3390/antiox12061266. Antioxidants (Basel). 2023. PMID: 37371996 Free PMC article. Review.
References
-
- Storey K. Turning down the fires of life: Metabolic regulation of hibernation and estivation. Mol. Mech. Metab. Arrest. 2001;126:S90. doi: 10.1016/S0305-0491(00)80178-9. - DOI
-
- Hermes-Lima M., Storey J.M., Storey K.B. Antioxidant defenses and animal adaptation to oxygen availability during environmental stress. In: Storey K.B., Storey J.M., editors. Cell and Molecular Responses to Stress. Volume 2—Protein Adaptations and Signal Transduction. Elsevier Press; Amsterdam, Germany: 2001. pp. 263–287.
-
- Ponder W.F., Lindberg D.R., Ponder J.M. Biology and Evolution of the Mollusca. Volume 1 CRC Press; Boca Raton, FL, USA: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

