Synthesis of TiO2 Nanobelt Bundles Decorated with TiO2 Nanoparticles and Aggregates and Their Use as Anode Materials for Lithium-Ion Batteries
- PMID: 36837943
- PMCID: PMC9961189
- DOI: 10.3390/mi14020243
Synthesis of TiO2 Nanobelt Bundles Decorated with TiO2 Nanoparticles and Aggregates and Their Use as Anode Materials for Lithium-Ion Batteries
Abstract
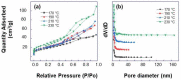
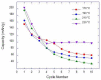
TiO2 nanobelt bundles decorated with TiO2 aggregates were prepared using an easy and scalable hydrothermal method at various temperatures (170, 190, 210, and 230 °C). It was demonstrated that the synthesis temperature is a key parameter to tune the number of aggregates on the nanobelt surface. Prepared TiO2 aggregates and nanobelt bundles were used to design anode materials in which the aggregates regulated the pore size and connectivity of the interconnected nanobelt bundle structure. A galvanostatic technique was employed for the electrochemical characterization of TiO2 samples. Using TiO2 as a model material due to its small volume change during the cycling of lithium-ion batteries (LIBs), the relationship between the morphology of the anode materials and the capacity retention of the LIBs on cycling is discussed. It was clearly found that the size and connectivity of the pores and the specific surface area had a striking impact on the Li insertion behavior, lithium storage capability, and cycling performance of the batteries. The initial irreversible capacity was shown to increase as the specific surface area increased. As the pore size increased, the ability of the mesoporous anatase to release strain was stronger, resulting in better cycling stability. The TiO2 powder prepared at a temperature of 230 °C displayed the highest discharge and charge capacities (203.3 mAh/g and 140.8 mAh/g) and good cycling stability.
Keywords: lithium-ion batteries; mesoporous TiO2; pores architecture; specific surface area.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Qu J., Ding J., Yuan N. The synthesis of four morphologies of TiO2 through temperature control and their electrochemical performance. Int. J. Elecrochem. Sci. 2015;10:8385–8394.
-
- Lu J., Chen Z., Pan F., Cui Y., Amine K. High-Performance anode materials for rechargeable lithium-ion batteries. Electrochem. Ener. Rev. 2018;1:35–53. doi: 10.1007/s41918-018-0001-4. - DOI
-
- Xiang C., Li M., Zhi M., Manivannan A., Wu N. Reduced graphene oxide/titanium dioxide composites for supercapacitor electrodes: Shape and coupling effects. J. Mater. Chem. 2012;22:19161. doi: 10.1039/c2jm33177b. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

