Highly-Sensitive, Label-Free Detection of Microorganisms and Viruses via Interferometric Reflectance Imaging Sensor
- PMID: 36837980
- PMCID: PMC9960798
- DOI: 10.3390/mi14020281
Highly-Sensitive, Label-Free Detection of Microorganisms and Viruses via Interferometric Reflectance Imaging Sensor
Abstract
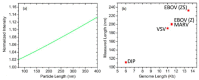
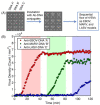
Pathogenic microorganisms and viruses can easily transfer from one host to another and cause disease in humans. The determination of these pathogens in a time- and cost-effective way is an extreme challenge for researchers. Rapid and label-free detection of pathogenic microorganisms and viruses is critical in ensuring rapid and appropriate treatment. Sensor technologies have shown considerable advancements in viral diagnostics, demonstrating their great potential for being fast and sensitive detection platforms. In this review, we present a summary of the use of an interferometric reflectance imaging sensor (IRIS) for the detection of microorganisms. We highlight low magnification modality of IRIS as an ensemble biomolecular mass measurement technique and high magnification modality for the digital detection of individual nanoparticles and viruses. We discuss the two different modalities of IRIS and their applications in the sensitive detection of microorganisms and viruses.
Keywords: biomass measurement; interferometric reflectance imaging sensor; nanoparticle detection; pathogenic microorganisms; sensitive detection; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Leonard P., Hearty S., Brennan J., Dunne L., Quinn J., Chakraborty T., O’Kennedy R. Advances in biosensors for detection of pathogens in food and water. Enzym. Microb. Technol. 2003;32:3–13. doi: 10.1016/S0141-0229(02)00232-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

