Impedance-Frequency Response of Closed Electrolytic Cells
- PMID: 36838068
- PMCID: PMC9963336
- DOI: 10.3390/mi14020368
Impedance-Frequency Response of Closed Electrolytic Cells
Abstract
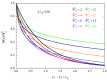
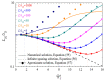
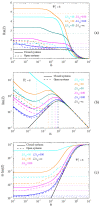
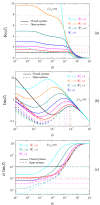
The electric AC response of electrolytic cells with DC bias is analyzed solving numerically the Poisson-Nernst-Planck equations and avoiding the commonly used infinite solution approximation. The results show the presence of an additional low-frequency dispersion process associated with the finite spacing of the electrodes. Moreover, we find that the condition of fixed ionic content inside the electrolytic cell has a strong bearing on both the steady-state and the frequency response. For example: the characteristic frequency of the high-frequency dispersion decreases when the DC potential increases and/or the electrode spacing decreases in the closed cell case, while it remains essentially insensitive on these changes for open cells. Finally, approximate analytic expressions for the dependences of the main parameters of both dispersion processes are also presented.
Keywords: Poisson-Nernst Planck equations; closed electrolytic cells; electrical impedance spectroscopy.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures

References
-
- Barsoukov E., Macdonald J.R. Impedance Spectroscopy: Theory, Experiment and Applications. Wiley-Interscience; New York, NY, USA: 2005.
-
- Bard A.J., Faulkner L.R. Electrochemical Methods: Fundamentals and Applications. John Wiley & Sons; Hoboken, NJ, USA: 2001.
-
- Brumleve T.R., Buck R.P. Numerical solution of the Nernst-Planck and Poisson equation system with applications to membrane electrochemistry and solid state physics. J. Electroanal. Chem. 1978;90:1–31. doi: 10.1016/S0022-0728(78)80137-5. - DOI
-
- Sorensen T.B., Compañ V. Complex permittivity of a conducting, dielectric layer containing arbitrary binary Nernst–Planck electrolytes with applications to polymer films and cellulose acetate membranes. J. Chem. Soc. Faraday Trans. 1995;91:4235–4250. doi: 10.1039/FT9959104235. - DOI
-
- Horno J., Moya A.A., González-Fernández C.F. Simulation and interpretation of electrochemical impedances using the network method. J. Electroanal. Chem. 1996;402:73–80. doi: 10.1016/0022-0728(95)04325-X. - DOI
LinkOut - more resources
Full Text Sources

