Organic Bioelectronics Development in Italy: A Review
- PMID: 36838160
- PMCID: PMC9966652
- DOI: 10.3390/mi14020460
Organic Bioelectronics Development in Italy: A Review
Abstract
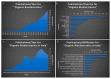
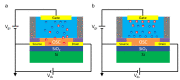
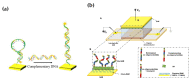
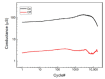
In recent years, studies concerning Organic Bioelectronics have had a constant growth due to the interest in disciplines such as medicine, biology and food safety in connecting the digital world with the biological one. Specific interests can be found in organic neuromorphic devices and organic transistor sensors, which are rapidly growing due to their low cost, high sensitivity and biocompatibility. This trend is evident in the literature produced in Italy, which is full of breakthrough papers concerning organic transistors-based sensors and organic neuromorphic devices. Therefore, this review focuses on analyzing the Italian production in this field, its trend and possible future evolutions.
Keywords: Organic Bioelectronics; biosensing; neuromorphic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- D’Angelo P., Tarabella G., Romeo A., Marasso S.L., Cocuzza M., Peruzzi C., Vurro D., Carotenuto G., Iannotta S. Nanomolar Detection of the Antitumor Drug Tamoxifen by Flexible Organic Electrochemical Devices. AIP Conf. Proc. 2018;1990:020015. doi: 10.1063/1.5047769. - DOI
-
- Battistoni S., Peruzzi C., Verna A., Marasso S.L., Cocuzza M., Erokhin V., Iannotta S. Synaptic Response in Organic Electrochemical Transistor Gated by a Graphene Electrode. Flex. Print. Electron. 2019;4:044002. doi: 10.1088/2058-8585/ab4dce. - DOI
-
- Sajapin R., Vurro D., D’Angelo P., Tarabella G., Marasso S.L., Cocuzza M., Botti M., Buttrini M., Calderaro A., Berzina T., et al. Aerosol Jet Printed Organic Memristive Microdevices Based on a Chitosan: PANI Composite Conductive Channel. ACS Appl. Electron. Mater. 2022;4:5875–5883. doi: 10.1021/acsaelm.2c01047. - DOI
-
- Cavallo A., Losi P., Buscemi M., Al Kayal T., Beccatelli M., Soldani G., Coppedè N. Biocompatible Organic Electrochemical Transistor on Polymeric Scaffold for Wound Healing Monitoring. Flex. Print. Electron. 2022;7:035009. doi: 10.1088/2058-8585/ac84ec. - DOI
-
- Gentile F., Vurro F., Janni M., Manfredi R., Cellini F., Petrozza A., Zappettini A., Coppedè N. A Biomimetic, Biocompatible OECT Sensor for the Real-Time Measurement of Concentration and Saturation of Ions in Plant Sap. Adv. Electron. Mater. 2022;8:200092. doi: 10.1002/aelm.202200092. - DOI
Publication types
LinkOut - more resources
Full Text Sources

