Strategies for the Voltammetric Detection of Loop-Mediated Isothermal Amplification
- PMID: 36838172
- PMCID: PMC9960872
- DOI: 10.3390/mi14020472
Strategies for the Voltammetric Detection of Loop-Mediated Isothermal Amplification
Abstract
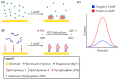
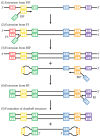
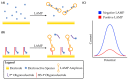
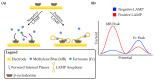
Loop-mediated isothermal amplification (LAMP) is rapidly developing into an important tool for the point-of-use detection of pathogens for both clinical and environmental samples, largely due to its sensitivity, rapidity, and adaptability to portable devices. Many methods are used to monitor LAMP, but not all are amenable to point-of-use applications. Common methods such as fluorescence often require bulky equipment, whereas colorimetric and turbidimetric methods can lack sensitivity. Electrochemical biosensors are becoming increasingly important for these applications due to their potential for low cost, high sensitivity, and capacity for miniaturization into integrated devices. This review provides an overview of the use of voltammetric sensors for monitoring LAMP, with a specific focus on how electroactive species are used to interface between the biochemical products of the LAMP reaction and the voltammetric sensor. Various strategies for the voltammetric detection of DNA amplicons as well as pyrophosphate and protons released during LAMP are presented, ranging from direct DNA binding by electroactive species to the creative use of pyrophosphate-detecting aptamers and pH-sensitive oligonucleotide structures. Hurdles for adapting these devices to point-of-use applications are also discussed.
Keywords: electrochemical biosensors; loop-mediated isothermal amplification; voltammetry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Detection methods for results of a loop-mediated isothermal amplification of DNA.].Klin Lab Diagn. 2020;65(1):67-72. doi: 10.18821/0869-2084-2020-65-1-67-72. Klin Lab Diagn. 2020. PMID: 32155010 Review. Russian.
-
Principles and Applications of Loop-Mediated Isothermal Amplification to Point-of-Care Tests.Biosensors (Basel). 2022 Oct 10;12(10):857. doi: 10.3390/bios12100857. Biosensors (Basel). 2022. PMID: 36290994 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification for Detection of Plant Pathogens in Wheat (Triticum aestivum).Front Plant Sci. 2022 Mar 15;13:857673. doi: 10.3389/fpls.2022.857673. eCollection 2022. Front Plant Sci. 2022. PMID: 35371152 Free PMC article. Review.
-
High-Surety Isothermal Amplification and Detection of SARS-CoV-2.mSphere. 2021 May 19;6(3):e00911-20. doi: 10.1128/mSphere.00911-20. mSphere. 2021. PMID: 34011690 Free PMC article.
-
Multiplexed Detection of Pathogens Using Solid-Phase Loop-Mediated Isothermal Amplification on a Supercritical Angle Fluorescence Array for Point-of-Care Applications.ACS Sens. 2022 Nov 25;7(11):3343-3351. doi: 10.1021/acssensors.2c01337. Epub 2022 Oct 25. ACS Sens. 2022. PMID: 36284082
Cited by
-
Colorimetric and electrochemical analysis of DNAzyme-LAMP amplicons for the detection of Escherichia coli in food matrices.Sci Rep. 2024 Nov 22;14(1):28942. doi: 10.1038/s41598-024-80392-4. Sci Rep. 2024. PMID: 39578633 Free PMC article.
References
-
- Espy M.J., Uhl J.R., Sloan L.M., Buckwalter S.P., Jones M.F., Vetter E.A., Yao J.D.C., Wengenack N.L., Rosenblatt J.E., Cockerill F.R., et al. Real-Time PCR in Clinical Microbiology: Applications for Routine Laboratory Testing. Clin. Microbiol. Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

