The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool
- PMID: 36838249
- PMCID: PMC9961521
- DOI: 10.3390/microorganisms11020284
The Use of Rapid COVID-19 Antigen Test in the Emergency Department as a Decision-Support Tool
Abstract
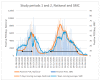
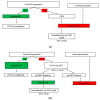
The emergency department (ED) is the initial point of contact between hospital staff and patients potentially infected with SARS-CoV-2, thus, prevention of inadvertent exposure to other patients is a top priority. We aimed to assess whether the introduction of antigen-detecting rapid diagnostic tests (Ag-RDTs) to the ED affected the likelihood of unwanted SARS-CoV-2 exposures. In this retrospective single-center study, we compared the rate of unwarranted exposure of uninfected adult ED patients to SARS-CoV-2 during two separate research periods; one before Ag-RDTs were introduced, and one with Ag-RDT used as a decision-support tool. The introduction of Ag-RDTs to the ED significantly decreased the relative risk of SARS-CoV-2-negative patients being incorrectly assigned to the COVID-19 designated site ("red ED"), by 97%. There was no increase in the risk of SARS-CoV-2-positive patients incorrectly assigned to the COVID-19-free site ("green ED"). In addition, duration of ED admission was reduced in both the red and the green ED. Therefore, implementing the Ag-RDT-based triage protocol proved beneficial in preventing potential COVID-19 nosocomial transmission.
Keywords: COVID-19; SARS-CoV-19; antigen; decision-making; emergency department; rapid tests; triage; triage protocol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Impaired performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests at elevated and low temperatures.J Clin Virol. 2021 May;138:104796. doi: 10.1016/j.jcv.2021.104796. Epub 2021 Mar 16. J Clin Virol. 2021. PMID: 33773413 Free PMC article.
-
Rapid Antigen Test Combined with Chest Computed Tomography to Rule Out COVID-19 in Patients Admitted to the Emergency Department.J Clin Med. 2021 Aug 4;10(16):3455. doi: 10.3390/jcm10163455. J Clin Med. 2021. PMID: 34441750 Free PMC article.
-
Bean Extract-Based Gargle for Efficient Diagnosis of Active COVID-19 Infection Using Rapid Antigen Tests.Microbiol Spectr. 2022 Feb 23;10(1):e0161421. doi: 10.1128/spectrum.01614-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171037 Free PMC article.
-
[Evaluation of two COVID-19 antigenic diagnostic tests: BIOSYNEX® COVID-19 Ag BSS and BIOSYNEX® COVID-19 Ag + BSS compared to AmpliQuick® SARS-CoV-2 PCR].Pan Afr Med J. 2021 Aug 6;39:228. doi: 10.11604/pamj.2021.39.228.30752. eCollection 2021. Pan Afr Med J. 2021. PMID: 34630840 Free PMC article. French.
-
Implementing COVID-19 (SARS-CoV-2) Rapid Diagnostic Tests in Sub-Saharan Africa: A Review.Front Med (Lausanne). 2020 Oct 30;7:557797. doi: 10.3389/fmed.2020.557797. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33195307 Free PMC article. Review.
References
-
- Israel Ministry of Health COVID-19 Data Dashboard. 29 June 2022. [(accessed on 29 June 2022)]; Available online: https://datadashboard.health.gov.il/COVID-19/general.
-
- World Health Organization Laboratory Testing of 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases: Interim Guidance. Updated 17 January 2020. [(accessed on 13 February 2022)]. Available online: https://apps.who.int/iris/handle/10665/330676.
LinkOut - more resources
Full Text Sources
Miscellaneous

