eDNA Provides a Scaffold for Autoaggregation of B. subtilis in Bacterioplankton Suspension
- PMID: 36838297
- PMCID: PMC9966259
- DOI: 10.3390/microorganisms11020332
eDNA Provides a Scaffold for Autoaggregation of B. subtilis in Bacterioplankton Suspension
Abstract
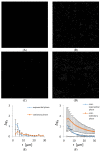
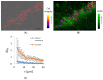
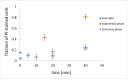
The self-binding of bacterial cells, or autoaggregation, is, together with surface colonization, one of the first steps in the formation of a mature biofilm. In this work, the autoaggregation of B. subtilis in dilute bacterial suspensions was studied. The dynamics of cell lysis, eDNA release, and bacterial autoaggregate assembly were determined and related to the spatial autocorrelation of bacterial cells in dilute planktonic bacterial suspensions. The non-random distribution of cells was associated with an eDNA network, which stabilized the initial bacterial cell-cell aggregates. Upon the addition of DNase I, the aggregates were dispersed. The release of eDNA during cell lysis allows for the entrapment of bacterial drifters at a radius several times the size of the dying bacteria. The size of bacterial aggregates increased from 2 to about 100 μm in diameter in dilute bacterial suspensions. The results suggest that B. subtilis cells form previously unnoticed continuum of autoaggregate structures during planktonic growth.
Keywords: B. subtilis; autoaggregates; biofilm; eDNA; plankton.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Bossier P., Verstraete W. Triggers for microbial aggregation in activated sludge? Appl. Microbiol. Biotechnol. 1996;45:1–6. doi: 10.1007/s002530050640. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

