Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses
- PMID: 36838310
- PMCID: PMC9966117
- DOI: 10.3390/microorganisms11020346
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Focus on the Pathophysiological and Diagnostic Role of Viruses
Abstract
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a heterogeneous, multiorgan and potentially life-threatening drug-hypersensitivity reaction (DHR) that occurs several days or weeks after drug initiation or discontinuation. DHRs constitute an emerging issue for public health, due to population aging, growing multi-organ morbidity, and subsequent enhanced drug prescriptions. DRESS has more consistently been associated with anticonvulsants, allopurinol and antibiotics, such as sulphonamides and vancomycin, although new drugs are increasingly reported as culprit agents. Reactivation of latent infectious agents such as viruses (especially Herpesviridae) plays a key role in prompting and sustaining aberrant T-cell and eosinophil responses to drugs and pathogens, ultimately causing organ damage. However, the boundaries of the impact of viral agents in the pathophysiology of DRESS are still ill-defined. Along with growing awareness of the multifaceted aspects of immune perturbation caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the ongoing SARS-CoV-2-related disease (COVID-19) pandemic, novel interest has been sparked towards DRESS and the potential interactions among antiviral and anti-drug inflammatory responses. In this review, we summarised the most recent evidence on pathophysiological mechanisms, diagnostic approaches, and clinical management of DRESS with the aim of increasing awareness on this syndrome and possibly suggesting clues for future research in this field.
Keywords: DRESS; T-cells; eosinophils; herpesvirus; reaction; viral reactivation; virus.
Conflict of interest statement
The authors declare no conflict of interest in connection with this paper.
Figures

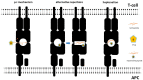
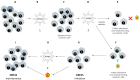
References
-
- Park C.S., Kim T.B., Kim S.L., Kim J.Y., Yang K.A., Bae Y.J., Cho Y.S., Moon H.B. The Use of an Electronic Medical Record System for Mandatory Reporting of Drug Hypersensitivity Reactions Has Been Shown to Improve the Management of Patients in the University Hospital in Korea. Pharmacoepidemiol. Drug Saf. 2008;17:919–925. doi: 10.1002/pds.1612. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

