Development of A Rapid, Low-Cost Portable Detection Assay for Enterococci in Wastewater and Environmental Waters
- PMID: 36838346
- PMCID: PMC9960780
- DOI: 10.3390/microorganisms11020381
Development of A Rapid, Low-Cost Portable Detection Assay for Enterococci in Wastewater and Environmental Waters
Abstract
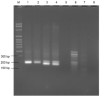
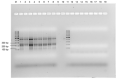
Waterborne diseases are known as a leading cause of illness and death in both developing and developed countries. Several pathogens can be present in contaminated water, particularly waters containing faecal material; however, routine monitoring of all pathogens is not currently possible. Enterococcus faecalis, which is present in the microflora of human and animals has been used as a faecal indicator in water due to its abundance in surface water and soil. Accurate and fast detection methods are critical for the effective monitoring of E. faecalis in the environment. Although conventional and current molecular detection techniques provide sufficient sensitivity, specificity and throughput, their use is hampered by the long waiting period (1-6 days) to obtain results, the need for expensive laboratory equipment, skilled personnel, and cold-chain storage. Therefore, this study aimed to develop a detection system for E. faecalis that would be simple, rapid, and low-cost, using an isothermal DNA amplification assay called recombinase polymerase amplification (RPA), integrated with a lateral flow assay (LFA). The assay was found to be 100% selective for E. faecalis and capable of detecting rates as low as 2.8 × 103 cells per 100 mL from water and wastewater, and 2.8 × 104 cells per 100 mL from saline water. The assay was completed in approximately 30 min using one constant temperature (38 °C). In addition, this study demonstrated the quantitation of E. faecalis using a lateral flow strip reader for the first time, enhancing the potential use of RPA assay for the enumeration of E. faecalis in wastewater and heavily contaminated environmental waters, surface water, and wastewater. However, the sensitivity of the RPA-LFA assay for the detection of E. faecalis in tap water, saline water and in wastewater was 10-1000 times lower than that of the Enterolert-E test, depending on the water quality. Nevertheless, with further improvements, this low-cost RPA-LFA may be suitable to be used at the point-of-need (PON) if conjugated with a rapid field-deployable DNA extraction method.
Keywords: Enterococcus faecalis; lateral flow assay; recombinase polymerase amplification; wastewater; water contamination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Rapid and specific detection of Enterococcus faecalis with a visualized isothermal amplification method.Front Cell Infect Microbiol. 2022 Sep 12;12:991849. doi: 10.3389/fcimb.2022.991849. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36171761 Free PMC article.
-
Utilization of recombinase polymerase amplification combined with a lateral flow strip for detection of Perkinsus beihaiensis in the oyster Crassostrea hongkongensis.Parasit Vectors. 2019 Jul 24;12(1):360. doi: 10.1186/s13071-019-3624-3. Parasit Vectors. 2019. PMID: 31340841 Free PMC article.
-
A loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Enterococcus spp. in water.Water Res. 2017 Oct 1;122:62-69. doi: 10.1016/j.watres.2017.05.023. Epub 2017 May 13. Water Res. 2017. PMID: 28591662
-
One-pot platform for rapid detecting virus utilizing recombinase polymerase amplification and CRISPR/Cas12a.Appl Microbiol Biotechnol. 2022 Jun;106(12):4607-4616. doi: 10.1007/s00253-022-12015-9. Epub 2022 Jun 16. Appl Microbiol Biotechnol. 2022. PMID: 35708748 Free PMC article.
-
Development of a recombinase polymerase based isothermal amplification combined with lateral flow assay (HLB-RPA-LFA) for rapid detection of "Candidatus Liberibacter asiaticus".PLoS One. 2018 Dec 12;13(12):e0208530. doi: 10.1371/journal.pone.0208530. eCollection 2018. PLoS One. 2018. PMID: 30540789 Free PMC article.
Cited by
-
A Portable UV-LED/RGB Sensor for Real-Time Bacteriological Water Quality Monitoring Using ML-Based MPN Estimation.Biosensors (Basel). 2025 Apr 30;15(5):284. doi: 10.3390/bios15050284. Biosensors (Basel). 2025. PMID: 40422022 Free PMC article.
-
Development of a Magnetic Bead-Based Method for Specific Detection of Enterococcus faecalis Using C-Terminal Domain of ECP3 Phage Endolysin.J Microbiol Biotechnol. 2023 Jul 28;33(7):964-972. doi: 10.4014/jmb.2302.02033. Epub 2023 Apr 20. J Microbiol Biotechnol. 2023. PMID: 37164751 Free PMC article.
-
Bacterivorous Ciliate Tetrahymena pyriformis Facilitates vanA Antibiotic Resistance Gene Transfer in Enterococcus faecalis.Antibiotics (Basel). 2025 Apr 28;14(5):448. doi: 10.3390/antibiotics14050448. Antibiotics (Basel). 2025. PMID: 40426515 Free PMC article.
References
-
- WHO . Drinking-Water. WHO; Geneva, Switzerland: 2022.
-
- Salgot M., Huertas E., Weber S., Dott W., Hollender J. Wastewater reuse and risk: Definition of key objectives. Desalination. 2006;187:29–40. doi: 10.1016/j.desal.2005.04.065. - DOI
-
- Fernandes L.S., Galvão A., Santos R., Monteiro S. Impact of water reuse on agricultural practices and human health. Environ. Res. 2023;216:114762. - PubMed
-
- Alegbeleye O., Sant’Ana A.S. Microbiological quality of irrigation water for cultivation of fruits and vegetables: An overview of available guidelines, water testing strategies and some factors that influence compliance. Environ. Res. 2022;220:114771. doi: 10.1016/j.envres.2022.114771. - DOI - PubMed
-
- Uyttendaele M., Jaykus L.A., Amoah P., Chiodini A., Cunliffe D., Jacxsens L., Holvoet K., Korsten L., Lau M., McClure P. Microbial hazards in irrigation water: Standards, norms, and testing to manage use of water in fresh produce primary production. Compr. Rev. Food Sci. Food Saf. 2015;14:336–356. doi: 10.1111/1541-4337.12133. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

