Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence
- PMID: 36838359
- PMCID: PMC9960997
- DOI: 10.3390/microorganisms11020394
Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence
Abstract
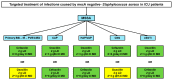
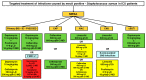
(1) Introduction: To develop evidence-based algorithms for targeted antibiotic therapy of infections caused by Staphylococcus aureus in critically ill adult patients. (2) Methods: A multidisciplinary team of four experts had several rounds of assessment for developing algorithms concerning targeted antimicrobial therapy of severe infections caused by Staphylococcus aureus in critically ill patients. The literature search was performed by a researcher on PubMed-MEDLINE (until August 2022) to provide evidence for supporting therapeutic choices. Quality and strength of evidence was established according to a hierarchical scale of the study design. Two different algorithms were created, one for methicillin-susceptible Staphylococcus aureus (MSSA) and the other for methicillin-resistant Staphylococcus aureus (MRSA). The therapeutic options were categorized for each different site of infection and were selected also on the basis of pharmacokinetic/pharmacodynamic features. (3) Results: Cefazolin or oxacillin were the agents proposed for all of the different types of severe MSSA infections. The proposed targeted therapies for severe MRSA infections were different according to the infection site: daptomycin plus fosfomycin or ceftaroline or ceftobiprole for bloodstream infections, infective endocarditis, and/or infections associated with intracardiac/intravascular devices; ceftaroline or ceftobiprole for community-acquired pneumonia; linezolid alone or plus fosfomycin for infection-related ventilator-associated complications or for central nervous system infections; daptomycin alone or plus clindamycin for necrotizing skin and soft tissue infections. (4) Conclusions: We are confident that targeted therapies based on scientific evidence and optimization of the pharmacokinetic/pharmacodynamic features of antibiotic monotherapy or combo therapy may represent valuable strategies for treating MSSA and MRSA infections.
Keywords: MRSA; MSSA; critically ill patients; multidisciplinary taskforce; targeted antibiotic therapy.
Conflict of interest statement
M.G. received personal fees from Angelini; B.V participated in advisory boards and in the speaker’s bureau for and received research contracts, contributions, and study events from Abbott, Accelerate Diagnostics, Ada, Alifax, Angelini, Becton Dickinson, Bellco, Biomerieux, Biotest, Cepheid, Correvio, Gilead, Menarini, MSD Italia, Nordic Pharma, Pfizer, Shionogi, and Thermo Fisher Scientific; G.M.R participated in advisory boards and the speaker’s bureau for and received research contracts, contributions, and travel grants from Accelerate, Angelini, Arrow, Beckman Biomedical Service, Coulter, Becton-Dickinson, bioMérieux, Cepheid, Hain Life Sciences, Menarini, Meridian, MSD, Nordic Pharma, Pfizer, Qiagen, Q-linea, Qpex, Quidel, Qvella, Roche, Seegene, Set-Lance, Shionogi, Symcel, ThermoFisher, VenatorX, and Zambon; F.P participated in the speaker’s bureau for Angelini, Basilea Pharmaceutica, Gilead, Hikma, Merck Sharp & Dohme, Nordic Pharma, Pfizer, and Sanofi Aventis and participated in the advisory board for Angelini, Basilea Pharmaceutica, Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, Novartis, Pfizer, and Thermo-Fisher. P.V has served as a consultant for Biomerieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx and received payment for serving on the speaker’s bureau for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer. The authors report no other conflicts of interest in this work.
Figures
References
-
- Khader K., Thomas A., Huskins W.C., Stevens V., Keegan L.T., Visnovsky L., Samore M.H. Effectiveness of Contact Precautions to Prevent Transmission of Methicillin-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococci in Intensive Care Units. Clin. Infect. Dis. 2021;72:S42–S49. doi: 10.1093/cid/ciaa1603. - DOI - PMC - PubMed
-
- Vincent J.-L., Sakr Y., Singer M., Martin-Loeches I., Machado F.R., Marshall J.C., Finfer S., Pelosi P., Brazzi L., Aditianingsih D., et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA. 2020;323:1478–1487. doi: 10.1001/jama.2020.2717. - DOI - PMC - PubMed
-
- European Centre for Disease Prevention and Control Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2020. [(accessed on 31 October 2022)]. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance....
LinkOut - more resources
Full Text Sources

