A Healthy Vaginal Microbiota Remains Stable during Oral Probiotic Supplementation: A Randomised Controlled Trial
- PMID: 36838464
- PMCID: PMC9961720
- DOI: 10.3390/microorganisms11020499
A Healthy Vaginal Microbiota Remains Stable during Oral Probiotic Supplementation: A Randomised Controlled Trial
Abstract
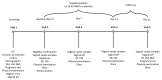
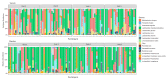
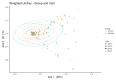
The primary objective of this randomised, placebo-controlled, triple-blind study was to assess whether orally consumed Lactobacillus acidophilus La-14 (La-14) and Lacticaseibacillus rhamnosus HN001 (HN001) colonise a healthy human vagina. Furthermore, potential effects on vaginal microbiota and immune markers were explored. Fifty women devoid of vaginal complaints (Nugent score 0-3 and vaginal pH ≤ 4.5) were randomised into a 2-week intervention with either La-14 and HN001 as the verum product or a comparable placebo. Vaginal swab samples were collected at baseline, after one and two weeks of intervention, and after a one-week follow-up, for assessing colonisation of the supplemented lactobacilli, vaginal microbiota, and six specific immune markers. Colonisation of L. acidophilus and L. rhamnosus was not observed above the assay detection limit (5.29 and 5.11 log 10 genomes/swab for L. acidophilus and L. rhamnosus, respectively). Vaginal microbiotas remained stable and predominated by lactobacilli throughout the intervention, and vaginal pH remained optimal (at least 90% of participants in both groups had pH 4.0 or 4.5 throughout the study). Immune markers elafin and human β-defensin 3 (HBD-3) were significantly decreased in the verum group (p = 0.022 and p = 0.028, respectively) but did not correlate with any microbiota changes. Adverse events raised no safety concerns, and no undesired changes in the vaginal microbiota or immune markers were detected.
Keywords: Lacticaseibacillus rhamnosus; Lactobacillus acidophilus; immune markers; lactobacilli; microbiota; probiotics; vaginal colonisation.
Conflict of interest statement
The sponsors participated in the design of the study, in the collection, analyses, and interpretation of data, in the writing of the manuscript, and in the decision to publish the results. Authors A.L., R.A.-J., N.Y., N.D., K.E., A.H., M.J.L, S.D.F., A.I., A.C.O., J.M. and L.L. were employed by the sponsor Danisco Sweeteners Oy or another subsidiary of IFF at the time the study was conducted. T.P. and J.J. were employed by 4Pharma Limited at the time the study was conducted. G.C., the principal investigator, and K.B. were employed by CPS Research at the time the study was conducted.
Figures

References
-
- Das S., Bhattacharjee M.J., Mukherjee A.K., Khan M.R. Recent advances in understanding of multifaceted changes in the vaginal microenvironment: Implications in vaginal health and therapeutics. Crit. Rev. Microbiol. 2022:1–27. doi: 10.1080/1040841X.2022.2049696. online ahead of print . - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

