Broad-Spectrum Antimicrobial Activity of Oftasecur and Visuprime Ophthalmic Solutions
- PMID: 36838468
- PMCID: PMC9959165
- DOI: 10.3390/microorganisms11020503
Broad-Spectrum Antimicrobial Activity of Oftasecur and Visuprime Ophthalmic Solutions
Abstract
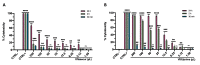
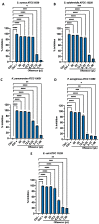
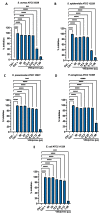
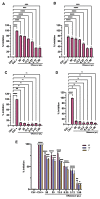
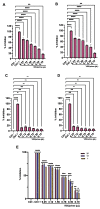
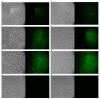
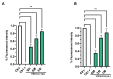
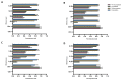
Due to the wide etiology of conjunctivitis, the expensive and time-consuming diagnosis requires new therapeutic strategies with broad-spectrum antimicrobial activity and nonselective mechanisms of action. In this context, eye drops could provide an alternative to conventional antimicrobial therapies. Here, we compare the antibacterial and antiviral activity of Oftasecur and Visuprime, commercially available ophthalmic solutions. Cytotoxicity assay was performed on Vero CCL-81 cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) test. Antibacterial efficacy was evaluated on Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae by disk diffusion, broth microdilution methods, and time-killing tests. Furthermore, the antiviral activity against HSV-1 was estimated by co-treatment, cell and viral pretreatment and post-treatment, via plaque reduction assay, fluorescence assessment (GFP-engineered HSV-1), and real-time PCR. After 24 h of exposure, Oftasecur and Visuprime showed a volume-inducing 50% of cytotoxicity of 125 and 15.8 μL, respectively Oftasecur and Visuprime induced 90% antibacterial activity in response to mean volume of 10.0 and 4.4 µL for Gram-positive and Gram-negative strains, respectively. Oftasecur exerted bactericidal action on both bacterial populations, while Visuprime was bacteriostatic on Gram-negative strains and slightly bactericidal on Gram-positive bacteria. A major impact on infectivity occurred by exposure of viral particles to the ophthalmic solutions. In detail, 50% of inhibition was verified by exposing the viral particles to 3.12 and 0.84 μL of Oftasecur and Visuprime, respectively, for 1 h. The reduction of the fluorescence and the expression of the viral genes confirmed the recorded antiviral activity. Due to their high antimicrobial efficiency, Oftasecur and Visuprime could represent a valid empirical strategy for the treatment of conjunctivitis.
Keywords: Gram-negative bacteria; Gram-positive bacteria; HSV-1; Oftasecur; Visuprime; antibacterial activity; antiviral potential; eye drops.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Antiseptics and the Ocular Surface: In Vitro Antimicrobial Activity and Effects on Conjunctival and Corneal Epithelial Cells of a New Liposomal Ocular Spray Containing Biosecur® Citrus Extract.Ophthalmol Ther. 2022 Jun;11(3):1067-1077. doi: 10.1007/s40123-022-00492-0. Epub 2022 Mar 13. Ophthalmol Ther. 2022. PMID: 35284982 Free PMC article.
-
Comparison Between Moxifloxacin and Chloramphenicol for the Treatment of Bacterial Eye Infections.Curr Ther Res Clin Exp. 2024 Feb 28;100:100740. doi: 10.1016/j.curtheres.2024.100740. eCollection 2024. Curr Ther Res Clin Exp. 2024. PMID: 38511104 Free PMC article.
-
In vitro antimicrobial activity of silver nanoparticles against selected Gram-negative and Gram-positive pathogens.Med Pharm Rep. 2024 Jul;97(3):280-297. doi: 10.15386/mpr-2750. Epub 2024 Jul 30. Med Pharm Rep. 2024. PMID: 39234464 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Literature review on the distribution characteristics and antimicrobial resistance of bacterial pathogens in neonatal sepsis.J Matern Fetal Neonatal Med. 2022 Mar;35(5):861-870. doi: 10.1080/14767058.2020.1732342. Epub 2020 Feb 26. J Matern Fetal Neonatal Med. 2022. PMID: 32102584 Review.
Cited by
-
Anti-Staphylococcal Biofilm Effects of a Liposome-Based Formulation Containing Citrus Polyphenols.Antibiotics (Basel). 2024 Mar 30;13(4):318. doi: 10.3390/antibiotics13040318. Antibiotics (Basel). 2024. PMID: 38666994 Free PMC article.
-
Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3.Int J Mol Sci. 2024 Aug 3;25(15):8480. doi: 10.3390/ijms25158480. Int J Mol Sci. 2024. PMID: 39126048 Free PMC article.
-
Case report: Emerging species in post-traumatic endophthalmitis: Acinetobacter johnsonii.Front Med (Lausanne). 2024 Aug 13;11:1406277. doi: 10.3389/fmed.2024.1406277. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39193018 Free PMC article.
References
-
- Hashmi M.F., Gurnani B., Benson S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Conjunctivitis.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

