Gut Microbiota as Well as Metabolomes of Wistar Rats Recover within Two Weeks after Doripenem Antibiotic Treatment
- PMID: 36838498
- PMCID: PMC9959319
- DOI: 10.3390/microorganisms11020533
Gut Microbiota as Well as Metabolomes of Wistar Rats Recover within Two Weeks after Doripenem Antibiotic Treatment
Abstract
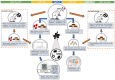
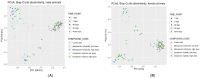
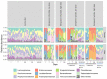
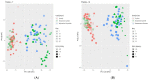
An understanding of the changes in gut microbiome composition and its associated metabolic functions is important to assess the potential implications thereof on host health. Thus, to elucidate the connection between the gut microbiome and the fecal and plasma metabolomes, two poorly bioavailable carbapenem antibiotics (doripenem and meropenem), were administered in a 28-day oral study to male and female Wistar rats. Additionally, the recovery of the gut microbiome and metabolomes in doripenem-exposed rats were studied one and two weeks after antibiotic treatment (i.e., doripenem-recovery groups). The 16S bacterial community analysis revealed an altered microbial population in all antibiotic treatments and a recovery of bacterial diversity in the doripenem-recovery groups. A similar pattern was observed in the fecal metabolomes of treated animals. In the recovery group, particularly after one week, an over-compensation was observed in fecal metabolites, as they were significantly changed in the opposite direction compared to previously changed metabolites upon 28 days of antibiotic exposure. Key plasma metabolites known to be diagnostic of antibiotic-induced microbial shifts, including indole derivatives, hippuric acid, and bile acids were also affected by the two carbapenems. Moreover, a unique increase in the levels of indole-3-acetic acid in plasma following meropenem treatment was observed. As was observed for the fecal metabolome, an overcompensation of plasma metabolites was observed in the recovery group. The data from this study provides insights into the connectivity of the microbiome and fecal and plasma metabolomes and demonstrates restoration post-antibiotic treatment not only for the microbiome but also for the metabolomes. The importance of overcompensation reactions for health needs further studies.
Keywords: carbapenems; gut microbiome; gut microbiota and metabolome recovery; metabolomics; repeated dose oral study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Connecting Gut Microbial Diversity with Plasma Metabolome and Fecal Bile Acid Changes Induced by the Antibiotics Tobramycin and Colistin Sulfate.Chem Res Toxicol. 2023 Apr 17;36(4):598-616. doi: 10.1021/acs.chemrestox.2c00316. Epub 2023 Mar 27. Chem Res Toxicol. 2023. PMID: 36972423
-
Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats.Arch Toxicol. 2017 Oct;91(10):3439-3454. doi: 10.1007/s00204-017-1949-2. Epub 2017 Mar 23. Arch Toxicol. 2017. PMID: 28337503
-
Antibiotic-Induced Changes in Microbiome-Related Metabolites and Bile Acids in Rat Plasma.Metabolites. 2020 Jun 11;10(6):242. doi: 10.3390/metabo10060242. Metabolites. 2020. PMID: 32545183 Free PMC article.
-
Investigating the gut microbiome and metabolome following treatment with artificial sweeteners acesulfame potassium and saccharin in young adult Wistar rats.Food Chem Toxicol. 2022 Jul;165:113123. doi: 10.1016/j.fct.2022.113123. Epub 2022 May 16. Food Chem Toxicol. 2022. PMID: 35588986
-
Analysis of metabolome changes in the bile acid pool in feces and plasma of antibiotic-treated rats.Toxicol Appl Pharmacol. 2019 Jan 15;363:79-87. doi: 10.1016/j.taap.2018.11.012. Epub 2018 Nov 28. Toxicol Appl Pharmacol. 2019. PMID: 30502395
Cited by
-
Absence of gut microbiota restoration following meropenem treatment in a systemic infection model.NPJ Antimicrob Resist. 2025 Jun 30;3(1):60. doi: 10.1038/s44259-025-00129-9. NPJ Antimicrob Resist. 2025. PMID: 40588535 Free PMC article.
References
-
- Jost L., DeVries P., Walla T., Greeney H., Chao A., Ricotta C. Partitioning diversity for conservation analyses. Divers. Distrib. 2010;16:65–76. doi: 10.1111/j.1472-4642.2009.00626.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

