Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties
- PMID: 36838561
- PMCID: PMC9963839
- DOI: 10.3390/molecules28041572
Two-Photon Absorption and Multiphoton Excited Fluorescence of Acetamide-Chalcone Derivatives: The Role of Dimethylamine Group on the Nonlinear Optical and Photophysical Properties
Abstract
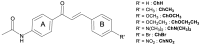
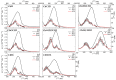
This work studied the effect of different electron-withdrawing and electron-donating groups on the linear and nonlinear optical properties of acetamide-chalcone derivatives. The results showed that the addition of the dimethylamine group led to a large fluorescence emission (71% of fluorescence quantum yield in DMSO solution) that can be triggered by two and three-photon excitations, which is essential for biological applications. Furthermore, dimethylamine also red-shifts the lower energy state by approximately 90 nm, increasing the two-photon absorption cross-section of the lower energy band by more than 100% compared with the other studied compounds. All compounds presented two-electronic states observed through one and two-photon absorption spectroscopy and confirmed by Quantum Chemistry Calculations (QCCs). QCC results were also used to model the experimental two-photon absorption cross-sectional spectrum by the Sum-Over-States (SOS) approach, revealing a dependence between the coupling of the ground state with the first excited state and the transition dipole moment between these states.
Keywords: SOS model; acetamide-chalcones; dimethylamine group; two and three-photon excited fluorescence emission; two-photon cross-section.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Abegão L.M., Santos F.A., Fonseca R.D., Barreiros A.L., Barreiros M.L., Alves P.B., Costa E.V., Souza G.B., Alencar M.A., Mendonça C.R., et al. Chalcone-based molecules: Experimental and theoretical studies on the two-photon absorption and molecular first hyperpolarizability. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;227:117772. doi: 10.1016/j.saa.2019.117772. - DOI - PubMed
-
- Manoel D.S., Pelosi A.G., Cocca L.H.Z., Almeida G.F., Sciuti L.F., Rodriguez R.D., Junior L.A., Lima R.S., Noda-Perez C., Martins F.T., et al. Second- and third-order nonlinear optical properties of mono-substituted terpenoid-like chalcones. J. Photochem. Photobiol. A Chem. 2022;429:113898. doi: 10.1016/j.jphotochem.2022.113898. - DOI
-
- Lemes S.R., Júnior L.A., Manoel D.D.S., de Sousa M.A.M., Fonseca R.D., Lima R.S., Noda-Perez C., Reis P.R.D.M., Cardoso C.G., Silveira-Lacerda E.D.P., et al. Optical properties and antiangiogenic activity of a chalcone derivate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;204:685–695. doi: 10.1016/j.saa.2018.06.099. - DOI - PubMed
-
- Abegão L.M., Fonseca R.D., Santos F.A., Souza G.B., Barreiros A.L.B., Barreiros M.L., Alencar M., Mendonça C.R., Silva D.L., De Boni L., et al. Second- and third-order nonlinear optical properties of unsubstituted and mono-substituted chalcones. Chem. Phys. Lett. 2016;648:91–96. doi: 10.1016/j.cplett.2016.02.009. - DOI
-
- da Costa R.G.M., Garcia R.D.Q., Fiuza R.M.d.R., Maqueira L., Pazini A., de Boni L., Limberger J. Synthesis, photophysical properties and aggregation-induced enhanced emission of bischalcone-benzothiadiazole and chalcone-benzothiadiazole hybrids. J. Lumin. 2021;239:118367. doi: 10.1016/j.jlumin.2021.118367. - DOI
MeSH terms
Substances
Grants and funding
- W911NF-21-1-0362/Army Research Laboratory
- FA9550-12-1-0028/Air Force Office of Scientific Research
- 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
- 2011/12399-0, 2015/20032-0 2016/20886-1 and 2018/11283-7/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 201410267001776 and 201710267000533/Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG)
LinkOut - more resources
Full Text Sources

