Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity
- PMID: 36838563
- PMCID: PMC9967035
- DOI: 10.3390/molecules28041576
Clinical Evaluation Based on a New Approach to Improve the Accuracy of 4β-Hydroxycholesterol Measurement as a Biomarker of CYP3A4 Activity
Abstract
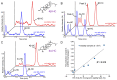
This study examines 4β-Hydroxycholesterol (4β-HC), which is considered to be a potential marker for the CYP3A4 induction of new chemical entities (NCEs) in drug development. To ensure the use of 4β-HC as a practical biomarker, it is necessary to accurately measure 4β-HC and demonstrate that CYP3A4 induction can be appropriately assessed, even for weak inducers. In clinical trials of NCEs, plasma is often collected with various anticoagulants, in some cases, the plasma is acidified, then stored for an extended period. In this study, we examined the effects of these manipulations on the measurement of 4β-HC, and based on the results, we optimized the plasma collection and storage protocols. We also found that a cholesterol oxidation product is formed when plasma is stored, and by monitoring the compound, we were able to identify when plasma was stored inappropriately. After evaluating the above, clinical drug-drug interaction (DDI) studies were conducted using two NCEs (novel retinoid-related orphan receptor γ antagonists). The weak CYP3A4 induction by the NCEs (which were determined based on a slight decline in the systemic exposure of a probe substrate (midazolam)), was detected by the significant increase in 4β-HC levels (more specifically, 4β-HC/total cholesterol ratios). Our new approach, based on monitoring a cholesterol oxidation product to identify plasma that is stored inappropriately, allowed for the accurate measurement of 4β-HC, and thus, it enabled the evaluation of weak CYP3A4 inducers in clinical studies without using a probe substrate.
Keywords: 4β-hydroxycholesterol; biomarkers; cholesterol oxidation; cytochrome P450 CYP3A4 inducers; drug–drug interaction study; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Björkhem-Bergman L., Bäckström T., Nylén H., Rönquist-Nii Y., Bredberg E., Andersson T.B., Bertilsson L., Diczfalusy U. Quinine compared to 4β-hydroxycholesterol and midazolam as markers for CYP3A induction by rifampicin. Drug Metab. Pharmacokinet. 2014;29:352–355. doi: 10.2133/dmpk.DMPK-13-SH-138. - DOI - PubMed
-
- Björkhem-Bergman L., Bäckström T., Nylén H., Rönquist-Nii Y., Bredberg E., Andersson T.B., Bertilsson L., Diczfalusy U. Comparison of endogenous 4β-hydroxycholesterol with midazolam as markers for CYP3A4 induction by rifampicin. Drug Metab. Dispos. 2013;41:1488–1493. doi: 10.1124/dmd.113.052316. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

