Enantioseparation of P-Stereogenic 1-Adamantyl Arylthiophosphonates and Their Stereospecific Transformation to 1-Adamantyl Aryl- H-phosphinates
- PMID: 36838571
- PMCID: PMC9966292
- DOI: 10.3390/molecules28041584
Enantioseparation of P-Stereogenic 1-Adamantyl Arylthiophosphonates and Their Stereospecific Transformation to 1-Adamantyl Aryl- H-phosphinates
Abstract
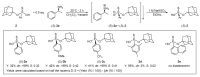
A focused library of 1-adamantyl arylthiophosphonates was prepared in racemic form. An enantioseparation method was developed for P-stereogenic thiophosphonates using (S)-1-phenylethylamine as the resolving agent. Under optimized conditions, three out of the five arylthiophosphonates were prepared in enantiopure form (ee > 99%). The subsequent desulfurization of optically active arylthiophosphonates gave the corresponding H-phosphinates without significant erosion of enantiomeric purity (ee = 95-98%). Hence, this reaction sequence can be considered an alternative method for the preparation of 1-adamantyl aryl-H-phopshinates. The absolute configuration of the (S)-1-adamantyl phenylphosphonothioic acid was assigned using single-crystal XRD and it allowed the confirmation that the removal of the P = S group proceeds with retention of configuration. The organocatalytic applicability of (S)-1-adamantyl phenylphosphonothioic acid was also evaluated as a P-stereogenic Brønsted acid.
Keywords: H-phosphinate; P-stereogenic; enantioseparation; organocatalysis; thiophosphonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



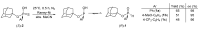

Similar articles
-
Enantioseparation of P-Stereogenic Secondary Phosphine Oxides and Their Stereospecific Transformation to Various Tertiary Phosphine Oxides and a Thiophosphinate.J Org Chem. 2021 Nov 5;86(21):14493-14507. doi: 10.1021/acs.joc.1c01364. Epub 2021 Oct 11. J Org Chem. 2021. PMID: 34633814 Free PMC article.
-
Stereospecific nucleophilic substitution of optically pure H-phosphinates: a general way for the preparation of chiral P-stereogenic phosphine oxides.J Am Chem Soc. 2008 Sep 24;130(38):12648-55. doi: 10.1021/ja804412k. Epub 2008 Aug 30. J Am Chem Soc. 2008. PMID: 18761459
-
Enantiopure cyclic O-substituted phenylphosphonothioic acid: synthesis and chirality-recognition ability.Chirality. 2011 Jul;23(6):438-48. doi: 10.1002/chir.20702. Epub 2009 Feb 19. Chirality. 2011. PMID: 19229959
-
Resolution of P-stereogenic P-heterocycles via the formation of diastereomeric molecular and coordination complexes (a review).Dalton Trans. 2016 Feb 7;45(5):1823-42. doi: 10.1039/c5dt02999f. Epub 2015 Nov 13. Dalton Trans. 2016. PMID: 26564410 Review.
-
Enantiomeric Resolution of (RS)-Naproxen and Application of (S)- Naproxen in the Direct and Indirect Enantioseparation of Racemic Compounds by Liquid Chromatography: A Review.Curr Med Chem. 2017;24(8):758-780. doi: 10.2174/0929867323666161129093052. Curr Med Chem. 2017. PMID: 27897117 Review.
References
-
- Kamer P.C.J., Van Leeuwen P.W.N.M., editors. Phosphorus(III)Ligands in Homogeneous Catalysis: Design and Synthesis. John Wiley & Sons; New York, NY, USA: 2012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

