A Novel Hybridization LC-MS/MS Methodology for Quantification of siRNA in Plasma, CSF and Tissue Samples
- PMID: 36838605
- PMCID: PMC9967190
- DOI: 10.3390/molecules28041618
A Novel Hybridization LC-MS/MS Methodology for Quantification of siRNA in Plasma, CSF and Tissue Samples
Abstract
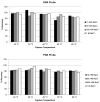
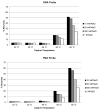
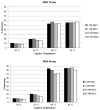
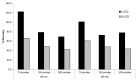
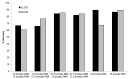
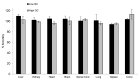
Therapeutic oligonucleotides, such as antisense oligonucleotide (ASO) and small interfering RNA (siRNA), are a new class of therapeutics rapidly growing in drug discovery and development. A sensitive and reliable method to quantify oligonucleotides in biological samples is critical to study their pharmacokinetic and pharmacodynamic properties. Hybridization LC-MS/MS was recently established as a highly sensitive and specific methodology for the quantification of single-stranded oligonucleotides, e.g., ASOs, in various biological matrices. However, there is no report of this methodology for the bioanalysis of double-stranded oligonucleotides (e.g., siRNA). In this work, we investigated hybridization LC-MS/MS methodology for the quantification of double-stranded oligonucleotides in biological samples using an siRNA compound, siRNA-01, as the test compound. The commonly used DNA capture probe and a new peptide nucleic acid (PNA) probe were compared for the hybridization extraction of siRNA-01 under different conditions. The PNA probe achieved better extraction recovery than the DNA probe, especially for high concentration samples, which may be due to its stronger hybridization affinity. The optimized hybridization method using the PNA probe was successfully qualified for the quantitation of siRNA-01 in monkey plasma, cerebrospinal fluid (CSF), and tissue homogenates over the range of 2.00-1000 ng/mL. This work is the first report of the hybridization LC-MS/MS methodology for the quantification of double-stranded oligonucleotides. The developed methodology will be applied to pharmacokinetic and toxicokinetic studies of siRNA-01. This novel methodology can also be used for the quantitative bioanalysis of other double-stranded oligonucleotides.
Keywords: LC-MS/MS; bioanalysis; double-stranded oligonucleotides; hybridization; quantitative; small interfering RNA (siRNA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

