Synthesis of 6-Alkynylated Purine-Containing DNA via On-Column Sonogashira Coupling and Investigation of Their Base-Pairing Properties
- PMID: 36838761
- PMCID: PMC9965804
- DOI: 10.3390/molecules28041766
Synthesis of 6-Alkynylated Purine-Containing DNA via On-Column Sonogashira Coupling and Investigation of Their Base-Pairing Properties
Abstract
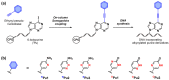
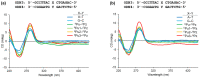
Synthetic unnatural base pairs have been proven to be attractive tools for the development of DNA-based biotechnology. Our group has very recently reported on alkynylated purine-pyridazine pairs, which exhibit selective and stable base-pairing via hydrogen bond formation between pseudo-nucleobases in the major groove of duplex DNA. In this study, we attempted to develop an on-column synthesis methodology of oligodeoxynucleotides (ODNs) containing alkynylated purine derivatives to systematically explore the relationship between the structure and the corresponding base-pairing ability. Through Sonogashira coupling of the ethynyl pseudo-nucleobases and CPG-bound ODNs containing 6-iodopurine, we have demonstrated the synthesis of the ODNs containing three NPu derivatives (NPu1, NPu2, NPu3) as well as three OPu derivatives (OPu1, OPu2, OPu3). The base-pairing properties of each alkynylated purine derivative revealed that the structures of pseudo-nucleobases influence the base pair stability and selectivity. Notably, we found that OPu1 bearing 2-pyrimidinone exhibits higher stability to the complementary NPz than the original OPu, thereby demonstrating the potential of the on-column strategy for convenient screening of the alkynylated purine derivatives with superior pairing ability.
Keywords: DNA; DNA major groove; Sonogashira coupling; hydrogen bonds; modified nucleosides; solid-phase synthesis; unnatural base pair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

