A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes
- PMID: 36838770
- PMCID: PMC9966098
- DOI: 10.3390/molecules28041781
A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes
Abstract
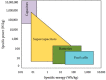
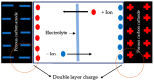
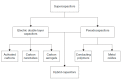
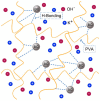
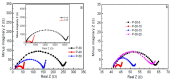
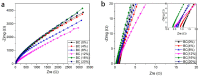
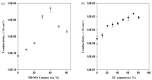
Presently, the rising concerns about the fossil fuel crisis and ecological deterioration have greatly affected the world economy and hence have attracted attention to the utilization of renewable energies. Among the renewable energy being developed, supercapacitors hold great promise in broad applications such as electric vehicles. Presently, the main challenge facing supercapacitors is the amount of energy stored. This, however, does not satisfy the increasing demand for higher energy storage devices, and therefore, intensive research is being undertaken to overcome the challenges of low energy density. The purpose of this review is to report on solid polymer electrolytes (SPEs) based on polyvinyl alcohol (PVA). The review discussed the PVA as a host polymer in SPEs followed by a discussion on the influence of conducting salts. The formation of SPEs as well as the ion transport mechanism in PVA SPEs were discussed. The application and development of PVA-based polymer electrolytes on supercapacitors and other energy storage devices were elucidated. The fundamentals of electrochemical characterization for analyzing the mechanism of supercapacitor applications, such as EIS, LSV and dielectric constant, are highlighted. Similarly, thermodynamic transport models of ions and their mechanism about temperature based on Arrhenius and Vogel-Tammann-Fulcher (VTF) are analyzed. Methods for enhancing the electrochemical performance of PVA-based SPEs were reported. Likely challenges facing the current electrolytes are well discussed. Finally, research directions to overcome the present challenges in producing SPEs are proposed. Therefore, this review is expected to be source material for other researchers concerned with the development of PVA-based SPE material.
Keywords: ionic conductivity; polyvinyl alcohol; solid polymer electrolytes; supercapacitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Burke A., Miller M. The power capability of ultracapacitors and lithium batteries for electric and hybrid vehicle applications. J. Power Sources. 2011;196:514–522. doi: 10.1016/j.jpowsour.2010.06.092. - DOI
-
- Mun Y., Jo C., Hyeon T., Lee J., Ha K.S., Jun K.W., Lee S.H., Hong S.W., Lee H.I., Yoon S., et al. Simple synthesis of hierarchically structured partially graphitized carbon by emulsion/blockcopolymer co-template method for high power supercapacitors. Carbon. 2013;64:391–402. doi: 10.1016/j.carbon.2013.07.092. - DOI
-
- Naoi K., Ishimoto S., Miyamoto J.I., Naoi W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012;11:9363–9373. doi: 10.1039/c2ee21675b. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

