Functionalized Sulfur-Containing Heterocyclic Analogs Induce Sub-G1 Arrest and Apoptotic Cell Death of Laryngeal Carcinoma In Vitro
- PMID: 36838844
- PMCID: PMC9963856
- DOI: 10.3390/molecules28041856
Functionalized Sulfur-Containing Heterocyclic Analogs Induce Sub-G1 Arrest and Apoptotic Cell Death of Laryngeal Carcinoma In Vitro
Abstract
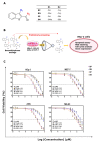
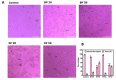
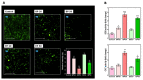
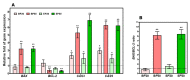
In this study, we speculate that the hydroxyl-containing benzo[b]thiophene analogs, 1-(3-hydroxybenzo[b]thiophen-2-yl) ethanone (BP) and 1-(3-hydroxybenzo[b]thiophen-2-yl) propan-1-one hydrate (EP), might possess antiproliferative activity against cancer cells. Hydroxyl-containing BP and EP show selectivity towards laryngeal cancer cells (HEp2), with IC50 values of 27.02 ± 1.23 and 35.26 ± 2.15 µM, respectively. The hydroxyl group present in the third position is responsible for the anticancer activity and is completely abrogated when the hydroxyl group is masked. BP and EP enhance the antioxidant enzyme activity and reduce the ROS production, which are correlated with the antiproliferative effect in HEp-2 cells. An increase in the BAX/BCL-2 ratio occurs during the BP and EP treatment and activates the caspase cascade, resulting in apoptosis stimulation. It also arrests the cells in the Sub-G1 phase, indicating the induction of apoptosis. The molecular docking and simulation studies predicted a strong interaction between BP and the CYP1A2 protein, which could aid in combinational therapy by enhancing the bioavailability of the drugs. BP and EP possess an antioxidant property with low antiproliferative effects (~5.18 µg/mL and ~7.8 µg/mL) as a standalone drug, therefore, they can be combined with other drugs for effective chemotherapy that might trigger the effect of pro-oxidant drug on healthy cells.
Keywords: anticancer; apoptosis; benzo[b]thiophene; laryngeal cancer; pharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Cancer Today. International Agency for Research on Cancer; Lyon, France: 2020. [(accessed on 27 March 2022)]. Global Cancer Observatory. Available online: https://gco.iarc.fr/today.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

