p-Xylene Oxidation to Terephthalic Acid: New Trends
- PMID: 36838910
- PMCID: PMC9961377
- DOI: 10.3390/molecules28041922
p-Xylene Oxidation to Terephthalic Acid: New Trends
Abstract
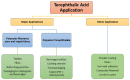
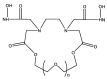
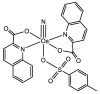
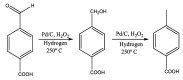
Large-scale terephthalic acid production from the oxidation of p-xylene is an especially important process in the polyester industry, as it is mainly used in polyethylene terephthalate (PET) manufacturing, a polymer that is widely used in fibers, films, and plastic products. This review presents and discusses catalytic advances and new trends in terephthalic acid production (since 2014), innovations in terephthalic acid purification processes, and simulations of reactors and reaction mechanisms.
Keywords: oxidation; p-xylene; polyethylene terephthalate; terephthalic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


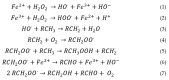







Similar articles
-
Hydrolysis of waste polyethylene terephthalate catalyzed by easily recyclable terephthalic acid.Waste Manag. 2021 Nov;135:267-274. doi: 10.1016/j.wasman.2021.09.009. Epub 2021 Sep 21. Waste Manag. 2021. PMID: 34555688
-
On the Diels-Alder approach to solely biomass-derived polyethylene terephthalate (PET): conversion of 2,5-dimethylfuran and acrolein into p-xylene.Chemistry. 2011 Oct 24;17(44):12452-7. doi: 10.1002/chem.201101580. Epub 2011 Sep 16. Chemistry. 2011. PMID: 21922576
-
Structure and function of the metagenomic plastic-degrading polyester hydrolase PHL7 bound to its product.Nat Commun. 2023 Apr 5;14(1):1905. doi: 10.1038/s41467-023-37415-x. Nat Commun. 2023. PMID: 37019924 Free PMC article.
-
p-Xylene oxidation to terephthalic acid: a literature review oriented toward process optimization and development.Chem Rev. 2013 Oct 9;113(10):7421-69. doi: 10.1021/cr300298j. Epub 2013 Jun 14. Chem Rev. 2013. PMID: 23767849 Review. No abstract available.
-
Microbial degradation and valorization of poly(ethylene terephthalate) (PET) monomers.World J Microbiol Biotechnol. 2022 Apr 15;38(5):89. doi: 10.1007/s11274-022-03270-z. World J Microbiol Biotechnol. 2022. PMID: 35426614 Review.
References
-
- Fadzil N.A.M., Rahim M.H.A., Maniam G.P. A brief review of para-xylene oxidation to terephthalic acid as a model of primary C–H bond activation. Chin. J. Catal. 2014;35:1641–1652. doi: 10.1016/S1872-2067(14)60193-5. - DOI
-
- PTA market value worldwide 2015-2029 | Statista. [(accessed on 14 December 2022)]. Available online: https://www.statista.com/statistics/1244421/global-market-value-purified...
-
- Global Purified Terephthalic Acid (PTA) Market to Grow to. [(accessed on 14 December 2022)]. Available online: https://www.globenewswire.com/en/news-release/2022/03/23/2408781/0/en/Gl....
-
- Purified Terephthalic Acid Market Size, Share, Trends & Forecast. [(accessed on 14 December 2022)]. Available online: https://www.verifiedmarketresearch.com/product/purified-terephthalic-aci...
-
- PTA global market volume 2015–2029 | Statista. [(accessed on 14 December 2022)]. Available online: https://www.statista.com/statistics/1245249/purified-terephthalic-acid-m...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

