The Impact of Fluorination on the Design of Histone Deacetylase Inhibitors
- PMID: 36838960
- PMCID: PMC9965134
- DOI: 10.3390/molecules28041973
The Impact of Fluorination on the Design of Histone Deacetylase Inhibitors
Abstract
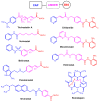
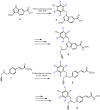
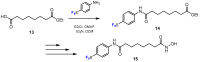
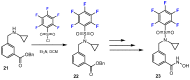
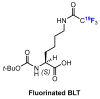
In recent years, histone deacetylases (HDACs) have emerged as promising targets in the treatment of cancer. The approach is to inhibit HDACs with drugs known as HDAC inhibitors (HDACis). Such HDACis are broadly classified according to their chemical structure, e.g., hydroxamic acids, benzamides, thiols, short-chain fatty acids, and cyclic peptides. Fluorination plays an important role in the medicinal-chemical design of new active representatives. As a result of the introduction of fluorine into the chemical structure, parameters such as potency or selectivity towards isoforms of HDACs can be increased. However, the impact of fluorination cannot always be clearly deduced. Nevertheless, a change in lipophilicity and, hence, solubility, as well as permeability, can influence the potency. The selectivity towards certain HDACs isoforms can be explained by special interactions of fluorinated compounds with the structure of the slightly different enzymes. Another aspect is that for a more detailed investigation of newly synthesized fluorine-containing active compounds, fluorination is often used for the purpose of labeling. Aside from the isotope 19F, which can be detected by nuclear magnetic resonance spectroscopy, the positron emission tomography of 18F plays a major role. However, to our best knowledge, a survey of the general effects of fluorination on HDACis development is lacking in the literature to date. Therefore, the aim of this review is to highlight the introduction of fluorine in the course of chemical synthesis and the impact on biological activity, using selected examples of recently developed fluorinated HDACis.
Keywords: fluorination; fluorine; fluorine-18; histone deacetylase (HDAC); histone deacetylase inhibitors (HDACis); positron emission tomography (PET); potency; selectivity; suberoylanilide hydroxamic acid (SAHA); vorinostat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




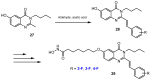



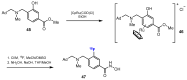
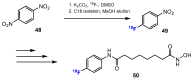
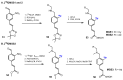









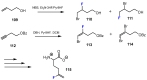
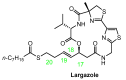
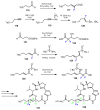


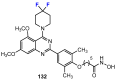


References
-
- Dalal N., Jalandra R., Sharma M., Prakash H., Makharia G.K., Solanki P.R., Singh R., Kumar A. Omics technologies for improved diagnosis and treatment of colorectal cancer: Technical advancement and major perspectives. Biomed. Pharmacother. 2020;131:110648. doi: 10.1016/j.biopha.2020.110648. - DOI - PubMed
-
- Steiner N., Schober P., Willenbacher W., Kircher B., Gunsilius E., Wolf D., Nachbaur D. Autologous and Allogeneic Stem Cell Transplantation as Salvage Treatment Options for Relapsed/Refractory Multiple Myeloma: A Single-center Experience over 20 Years. Anticancer Res. 2022;42:5825–5832. doi: 10.21873/anticanres.16090. - DOI - PubMed
-
- Steiner N., Göbel G., Mauser L., Mühlnikel L., Fischinger M., Künz T., Willenbacher W., Hetzenauer G., Rudzki J., Nussbaumer W., et al. Poor Mobilizers in Lymphoma but Not Myeloma Patients Had Significantly Poorer Progression-Free Survival after Autologous Stem Cell Transplantation: Results of a Large Retrospective, Single-Center Observational Study. Cancers. 2023;15:608. doi: 10.3390/cancers15030608. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

