Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration
- PMID: 36839138
- PMCID: PMC9965208
- DOI: 10.3390/nano13040770
Lamivudine and Zidovudine-Loaded Nanostructures: Green Chemistry Preparation for Pediatric Oral Administration
Abstract
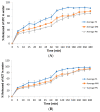
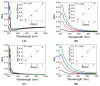
Here, we report on the development of lipid-based nanostructures containing zidovudine (1 mg/mL) and lamivudine (0.5 mg/mL) for oral administration in the pediatric population, eliminating the use of organic solvents, which is in accordance with green chemistry principles. The formulations were obtained by ultrasonication using monoolein (MN) or phytantriol (PN), which presented narrow size distributions with similar mean particle sizes (~150 nm) determined by laser diffraction. The zeta potential and the pH values of the formulations were around -4.0 mV and 6.0, respectively. MN presented a slightly higher incorporation rate compared to PN. Nanoemulsions were obtained when using monoolein, while cubosomes were obtained when using phytantriol, as confirmed by Small-Angle X-ray Scattering. The formulations enabled drug release control and protection against acid degradation. The drug incorporation was effective and the analyses using an electronic tongue indicated a difference in palatability between the nanotechnological samples in comparison with the drug solutions. In conclusion, PN was considered to have the strongest potential as a novel oral formulation for pediatric HIV treatment.
Keywords: children; cubosomes; green chemistry; nanoemulsion; oral administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Marques M.S., Lima L.A., Poletto F., Contri R.V., Kulkamp Guerreiro I.C. Nanotechnology for the Treatment of Paediatric Diseases: A Review. J. Drug. Deliv. Sci. Technol. 2022;75:103628. doi: 10.1016/j.jddst.2022.103628. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

