Improved Strength Recovery and Reduced Fatigue with Suppressed Plasma Myostatin Following Supplementation of a Vicia faba Hydrolysate, in a Healthy Male Population
- PMID: 36839344
- PMCID: PMC9967853
- DOI: 10.3390/nu15040986
Improved Strength Recovery and Reduced Fatigue with Suppressed Plasma Myostatin Following Supplementation of a Vicia faba Hydrolysate, in a Healthy Male Population
Abstract
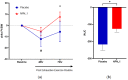
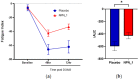
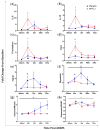
Delayed onset muscle soreness (DOMS) due to intense physical exertion can negatively impact contractility and performance. Previously, NPN_1 (PeptiStrong™), a Vicia faba hydrolysate derived from a protein concentrate discovered through artificial intelligence (AI), was preclinically shown to help maintain muscle health, indicating the potential to mediate the effect of DOMS and alter molecular markers of muscle damage to improve recovery and performance. A randomised double-blind placebo-controlled trial was conducted on 30 healthy male (30-45 years old) volunteers (NCT05159375). Following initial strength testing on day 0, subjects were administered either placebo or NPN_1 (2.4 g/day). On day 14, DOMS was induced using resistance exercise. Strength recovery and fatigue were measured after 48 and 72 h. Biomarker analysis was performed on blood samples collected prior to DOMS induction and 0, 2, 48 and 72 h post-DOMS induction. NPN_1 supplementation significantly improved strength recovery compared to placebo over the 72 h period post-resistance exercise (p = 0.027), measured by peak torque per bodyweight, but not at individual timepoints. Muscle fatigue was significantly reduced over the same 72 h period (p = 0.041), as was myostatin expression (p = 0.006). A concomitant increase in other acute markers regulating muscle protein synthesis, regeneration and myoblast differentiation was also observed. NPN_1 significantly improves strength recovery and restoration, reduces fatigue and positively modulates alterations in markers related to muscle homeostasis.
Keywords: Vicia faba; bioactive peptide; exercise; fatigue; hydrolysate; inflammation; muscle; performance; recovery; strength.
Conflict of interest statement
Co-authors employed by Nuritas, A.W., A.K., B.K., H.D. and N.K. Co-authors employed by SSC Sports Medicine, L.H. and A.F.M. Co-author employed by Munster Technological University, S.L. The project was financially supported by Nuritas Ltd. Nuritas and SSC Sports Medicine are responsible for the study design, data collection and analysis, decision to publish and preparation of the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

