Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine
- PMID: 36839400
- PMCID: PMC9967785
- DOI: 10.3390/nu15041042
Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine
Abstract
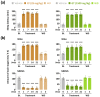
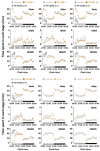
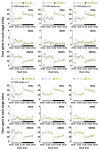
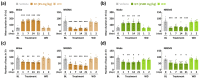
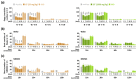
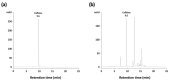
Wakefulness is defined as a state in which individuals can react to a change in situations. The number of people staying awake and compensating for lack of sleep has increased in recent years. Caffeine, a representative stimulant, is the most extensively consumed compound globally and is mainly consumed through coffee. Although green tea (Camellia sinensis L.) contains high caffeine content like coffee, its arousal-inducing effects have not yet been studied. In the present study, we aimed to identify the arousal-inducing effect of GT during a chronic administration period (three weeks) using analysis of sleep architecture. Treatment with GT (1500 mg/kg) significantly elevated the sleep latency and wakefulness throughout the treatment period, and chronic administration of GT consistently maintained an increase in wakefulness for up to 3 h. During the treatment period, the arousal-inducing effect of GT (1500 mg/kg) occurred without any change in the tolerance phenomenon or withdrawal symptoms, similar to that observed with caffeine (25 mg/kg). GT (1500 mg/kg) containing 95.6 mg/kg of caffeine did not produce a better arousal-inducing effect than caffeine at 25 mg/kg. These results indicate that the arousal-inducing effect of GT persisted for three weeks without adverse effects and that GT can control the arousal-inducing effects of caffeine due to the hypnotic effects of its other constituents.
Keywords: Camellia sinensis L.; arousal-inducing effect; caffeine; green tea ethanol extract; polysomnographic recording.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The polyphenol/caffeine ratio determines the arousal-inducing properties of the green tea ethanol extract.Food Funct. 2025 May 6;16(9):3694-3706. doi: 10.1039/d5fo00661a. Food Funct. 2025. PMID: 40245063
-
Arousal-Inducing Effect of Garcinia cambogia Peel Extract in Pentobarbital-Induced Sleep Test and Electroencephalographic Analysis.Nutrients. 2021 Aug 19;13(8):2845. doi: 10.3390/nu13082845. Nutrients. 2021. PMID: 34445005 Free PMC article.
-
A naturalistic investigation of the effects of day-long consumption of tea, coffee and water on alertness, sleep onset and sleep quality.Psychopharmacology (Berl). 2000 Apr;149(3):203-16. doi: 10.1007/s002130000383. Psychopharmacology (Berl). 2000. PMID: 10823400 Clinical Trial.
-
Toxicological effects of Camellia sinensis (green tea): A review.Phytother Res. 2018 Jul;32(7):1163-1180. doi: 10.1002/ptr.6063. Epub 2018 Mar 25. Phytother Res. 2018. PMID: 29575316 Review.
-
Caffeine ingestion and fluid balance: a review.J Hum Nutr Diet. 2003 Dec;16(6):411-20. doi: 10.1046/j.1365-277x.2003.00477.x. J Hum Nutr Diet. 2003. PMID: 19774754 Review.
Cited by
-
Investigation of Sleep Disorders and Related Influencing Factors Among the Elderly in Southeast Coastal Regions of China: A Cross-Sectional Survey Analysis.Clin Interv Aging. 2025 Feb 19;20:171-181. doi: 10.2147/CIA.S482763. eCollection 2025. Clin Interv Aging. 2025. PMID: 39990980 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

