Unravelling the Diversity of Microorganisms in Ticks from Australian Wildlife
- PMID: 36839425
- PMCID: PMC9967841
- DOI: 10.3390/pathogens12020153
Unravelling the Diversity of Microorganisms in Ticks from Australian Wildlife
Abstract
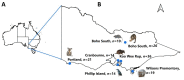
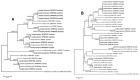
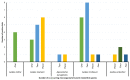
Ticks and tick-borne pathogens pose a significant threat to the health and welfare of humans and animals. Our knowledge about pathogens carried by ticks of Australian wildlife is limited. This study aimed to characterise ticks and tick-borne microorganisms from a range of wildlife species across six sites in Victoria, Australia. Following morphological and molecular characterisation (targeting 16S rRNA and cytochrome c oxidase I), tick DNA extracts (n = 140) were subjected to microfluidic real-time PCR-based screening for the detection of microorganisms and Rickettsia-specific real-time qPCRs. Five species of ixodid ticks were identified, including Aponomma auruginans, Ixodes (I.) antechini, I. kohlsi, I. tasmani and I. trichosuri. Phylogenetic analyses of 16S rRNA sequences of I. tasmani revealed two subclades, indicating a potential cryptic species. The microfluidic real-time PCR detected seven different microorganisms as a single (in 13/45 ticks) or multiple infections (27/45). The most common microorganisms detected were Apicomplexa (84.4%, 38/45) followed by Rickettsia sp. (55.6%, 25/45), Theileria sp. (22.2% 10/45), Bartonella sp. (17.8%, 8/45), Coxiella-like sp. (6.7%, 3/45), Hepatozoon sp. (2.2%, 1/45), and Ehrlichia sp. (2.2%, 1/45). Phylogenetic analyses of four Rickettsia loci showed that the Rickettsia isolates detected herein potentially belonged to a novel species of Rickettsia. This study demonstrated that ticks of Australian wildlife carry a diverse array of microorganisms. Given the direct and indirect human-wildlife-livestock interactions, there is a need to adopt a One Health approach for continuous surveillance of tick-associated pathogens/microorganisms to minimise the associated threats to animal and human health.
Keywords: Australia; Rickettsia; tick-borne pathogens; ticks; wildlife; zoonosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
The bacterial biome of ticks and their wildlife hosts at the urban-wildland interface.Microb Genom. 2021 Dec;7(12):000730. doi: 10.1099/mgen.0.000730. Microb Genom. 2021. PMID: 34913864 Free PMC article.
-
Genetic diversity of vector-borne pathogens in ixodid ticks infesting dogs from Pakistan with notes on Ehrlichia canis, Rickettsia raoultii and Dirofilaria immitis detection.Parasit Vectors. 2023 Jun 28;16(1):214. doi: 10.1186/s13071-023-05804-2. Parasit Vectors. 2023. PMID: 37381006 Free PMC article.
-
Novel Rickettsia and emergent tick-borne pathogens: A molecular survey of ticks and tick-borne pathogens in Shimba Hills National Reserve, Kenya.Ticks Tick Borne Dis. 2017 Feb;8(2):208-218. doi: 10.1016/j.ttbdis.2016.09.002. Epub 2016 Sep 5. Ticks Tick Borne Dis. 2017. PMID: 28011185
-
Human Tick-Borne Diseases in Australia.Front Cell Infect Microbiol. 2019 Jan 28;9:3. doi: 10.3389/fcimb.2019.00003. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30746341 Free PMC article. Review.
-
Are Colpodella Species Pathogenic? Nutrient Uptake and Approaches to Diagnose Infections.Pathogens. 2024 Jul 21;13(7):600. doi: 10.3390/pathogens13070600. Pathogens. 2024. PMID: 39057826 Free PMC article. Review.
Cited by
-
Causes of mortality and morbidity in the endangered southern brown bandicoot (Isoodon obesulus obesulus).Aust Vet J. 2025 Mar;103(3):138-148. doi: 10.1111/avj.13417. Epub 2025 Jan 26. Aust Vet J. 2025. PMID: 39865526 Free PMC article.
-
Dynamics of Infections in Cattle and Rhipicephalus microplus: A Preliminary Study.Pathogens. 2023 Jul 30;12(8):998. doi: 10.3390/pathogens12080998. Pathogens. 2023. PMID: 37623958 Free PMC article.
References
-
- Chapman A.D. Number of Living Species in Australia and the World. 2nd ed. Department of the Environment, Water, Heritage and the Arts; Canberra, Australia: 2009. Report for the Australian Biological Resources Study.
LinkOut - more resources
Full Text Sources

