Biomonitoring of Indoor Air Fungal or Chemical Toxins with Caenorhabditis elegans nematodes
- PMID: 36839433
- PMCID: PMC9964051
- DOI: 10.3390/pathogens12020161
Biomonitoring of Indoor Air Fungal or Chemical Toxins with Caenorhabditis elegans nematodes
Abstract
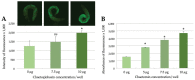
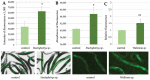
Bad indoor air quality due to toxins and other impurities can have a negative impact on human well-being, working capacity and health. Therefore, reliable methods to monitor the health risks associated with exposure to hazardous indoor air agents are needed. Here, we have used transgenic Caenorhabditis elegans nematode strains carrying stress-responsive fluorescent reporters and evaluated their ability to sense fungal or chemical toxins, especially those that are present in moisture-damaged buildings. Liquid-based or airborne exposure of nematodes to mycotoxins, chemical agents or damaged building materials reproducibly resulted in time- and dose-dependent fluorescent responses, which could be quantitated by either microscopy or spectrometry. Thus, the C. elegans nematodes present an easy, ethically acceptable and comprehensive in vivo model system to monitor the response of multicellular organisms to indoor air toxicity.
Keywords: C. elegans; biomonitoring; chemicals; fungi; microbes; toxins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures






References
-
- World Health Organization. Regional Office for Europe WHO Guidelines for Indoor Air Quality: Dampness and Mould. 2009. [(accessed on 22 July 2020)]. Available online: https://apps.who.int/iris/handle/10665/164348. - PubMed
-
- World Health Organization. Regional Office for Europe. European Centre for Environment and Health Effects of Air Pollution on Children’s Health and Development: A Review of the Evidence. 2005. [(accessed on 22 July 2020)]. Available online: https://apps.who.int/iris/handle/10665/107652.
-
- Eurostat Total Population Living in a Dwelling with a Leaking Roof, Damp Walls, Floors or Foundation, or Rot in Window Frames or Floor—EU-SILC Survey. 2020. [(accessed on 6 January 2021)]. Available online: https://ec.europa.eu/eurostat/en/web/products-datasets/-/ILC_MDHO01.
LinkOut - more resources
Full Text Sources
Research Materials

