3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals
- PMID: 36839636
- PMCID: PMC9967161
- DOI: 10.3390/pharmaceutics15020313
3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals
Abstract
3D printing technologies enable medicine customization adapted to patients' needs. There are several 3D printing techniques available, but majority of dosage forms and medical devices are printed using nozzle-based extrusion, laser-writing systems, and powder binder jetting. 3D printing has been demonstrated for a broad range of applications in development and targeting solid, semi-solid, and locally applied or implanted medicines. 3D-printed solid dosage forms allow the combination of one or more drugs within the same solid dosage form to improve patient compliance, facilitate deglutition, tailor the release profile, or fabricate new medicines for which no dosage form is available. Sustained-release 3D-printed implants, stents, and medical devices have been used mainly for joint replacement therapies, medical prostheses, and cardiovascular applications. Locally applied medicines, such as wound dressing, microneedles, and medicated contact lenses, have also been manufactured using 3D printing techniques. The challenge is to select the 3D printing technique most suitable for each application and the type of pharmaceutical ink that should be developed that possesses the required physicochemical and biological performance. The integration of biopharmaceuticals and nanotechnology-based drugs along with 3D printing ("nanoprinting") brings printed personalized nanomedicines within the most innovative perspectives for the coming years. Continuous manufacturing through the use of 3D-printed microfluidic chips facilitates their translation into clinical practice.
Keywords: 3D printing; FDM; PAM; SLA; SLS; bioprinting; fuse deposition modelling; microfluidic chip; nanomedicines; nanoparticle; peptide hydrogel; personalized medicines; pressure-assisted microsyringes; selective laser sintering; stereolithography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





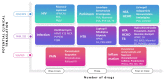

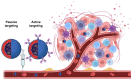
Comment in
-
3D printing - an alternative strategy for pediatric medicines.Expert Rev Clin Pharmacol. 2023 Jul-Dec;16(7):613-616. doi: 10.1080/17512433.2023.2233416. Epub 2023 Jul 5. Expert Rev Clin Pharmacol. 2023. PMID: 37408478 No abstract available.
References
-
- European Commission Personalised Medicines. [(accessed on 9 November 2022)]. Available online: https://research-and-innovation.ec.europa.eu/research-area/health/person....
-
- Giacomo G.D.A.D., Cury P.R., da Silva A.M., da Silva J.V., Ajzen S.A. Surgical guides for flapless dental implant placement and immediate definitive prosthesis installation by using selective laser melting and sintering for 3D metal and polymer printing: A clinical report. J. Prosthet. Dent. 2022 doi: 10.1016/j.prosdent.2022.05.034. - DOI - PubMed
-
- Li C.-H., Wu C.-H., Lin C.-L. Design of a patient-specific mandible reconstruction implant with dental prosthesis for metal 3D printing using integrated weighted topology optimization and finite element analysis. J. Mech. Behav. Biomed. Mater. 2020;105:103700. doi: 10.1016/j.jmbbm.2020.103700. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

